Regulation of human immunodeficiency virus type 1 Env-mediated membrane fusion by viral protease activity
- PMID: 14694135
- PMCID: PMC368813
- DOI: 10.1128/jvi.78.2.1026-1031.2004
Regulation of human immunodeficiency virus type 1 Env-mediated membrane fusion by viral protease activity
Abstract
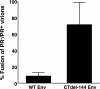
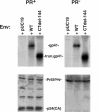
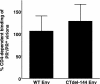
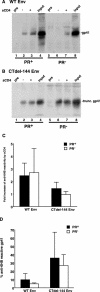
We and others have presented evidence for a direct interaction between the matrix (MA) domain of the human immunodeficiency virus type 1 (HIV-1) Gag protein and the cytoplasmic tail of the transmembrane envelope (Env) glycoprotein gp41. In addition, it has been postulated that the MA domain of Gag undergoes a conformational change following Gag processing, and the cytoplasmic tail of gp41 has been shown to modulate Env-mediated membrane fusion activity. Together, these results raise the possibility that the interaction between the gp41 cytoplasmic tail and MA is regulated by protease (PR)-mediated Gag processing, perhaps affecting Env function. To examine whether Gag processing affects Env-mediated fusion, we compared the ability of wild-type (WT) HIV-1 Env and a mutant lacking the gp41 cytoplasmic tail to induce fusion in the context of an active (PR(+)) or inactive (PR(-)) viral PR. We observed that PR(-) virions bearing WT Env displayed defects in cell-cell fusion. Impaired fusion did not appear to be due to differences in the levels of virion-associated Env, in CD4-dependent binding to target cells, or in the formation of the CD4-induced gp41 six-helix bundle. Interestingly, truncation of the gp41 cytoplasmic tail reversed the fusion defect. These results suggest that interactions between unprocessed Gag and the gp41 cytoplasmic tail suppress fusion.
Figures
Similar articles
-
Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation into virions.J Virol. 1996 Jan;70(1):341-51. doi: 10.1128/JVI.70.1.341-351.1996. J Virol. 1996. PMID: 8523546 Free PMC article.
-
Coupling of human immunodeficiency virus type 1 fusion to virion maturation: a novel role of the gp41 cytoplasmic tail.J Virol. 2004 Apr;78(7):3429-35. doi: 10.1128/jvi.78.7.3429-3435.2004. J Virol. 2004. PMID: 15016865 Free PMC article.
-
Downregulation of human immunodeficiency virus type 1 Gag expression by a gp41 cytoplasmic domain fusion protein.Virology. 2006 May 10;348(2):418-29. doi: 10.1016/j.virol.2006.01.010. Epub 2006 Feb 9. Virology. 2006. PMID: 16472834
-
HIV-1 gp41: mediator of fusion and target for inhibition.AIDS Rev. 2003 Oct-Dec;5(4):214-21. AIDS Rev. 2003. PMID: 15012000 Review.
-
HIV-1 replication.Somat Cell Mol Genet. 2001 Nov;26(1-6):13-33. doi: 10.1023/a:1021070512287. Somat Cell Mol Genet. 2001. PMID: 12465460 Review.
Cited by
-
Clustering and mobility of HIV-1 Env at viral assembly sites predict its propensity to induce cell-cell fusion.J Virol. 2013 Jul;87(13):7516-25. doi: 10.1128/JVI.00790-13. Epub 2013 May 1. J Virol. 2013. PMID: 23637402 Free PMC article.
-
HIV-1 assembly, release and maturation.Nat Rev Microbiol. 2015 Aug;13(8):484-96. doi: 10.1038/nrmicro3490. Epub 2015 Jun 29. Nat Rev Microbiol. 2015. PMID: 26119571 Free PMC article. Review.
-
HIV-1 Gag, Envelope, and Extracellular Determinants Cooperate To Regulate the Stability and Turnover of Virological Synapses.J Virol. 2016 Jun 24;90(14):6583-6597. doi: 10.1128/JVI.00600-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27170746 Free PMC article.
-
The HIV Env Glycoprotein Conformational States on Cells and Viruses.mBio. 2022 Apr 26;13(2):e0182521. doi: 10.1128/mbio.01825-21. Epub 2022 Mar 24. mBio. 2022. PMID: 35323042 Free PMC article. Review.
-
The Interplay between HIV-1 Gag Binding to the Plasma Membrane and Env Incorporation.Viruses. 2020 May 16;12(5):548. doi: 10.3390/v12050548. Viruses. 2020. PMID: 32429351 Free PMC article. Review.
References
-
- Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

