Gamma interferon-mediated inflammation is associated with lack of protection from intravaginal simian immunodeficiency virus SIVmac239 challenge in simian-human immunodeficiency virus 89.6-immunized rhesus macaques
- PMID: 14694116
- PMCID: PMC368742
- DOI: 10.1128/jvi.78.2.841-854.2004
Gamma interferon-mediated inflammation is associated with lack of protection from intravaginal simian immunodeficiency virus SIVmac239 challenge in simian-human immunodeficiency virus 89.6-immunized rhesus macaques
Abstract
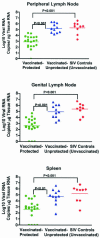
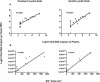
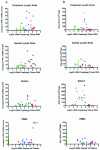
Although gamma interferon (IFN-gamma) is a key mediator of antiviral defenses, it is also a mediator of inflammation. As inflammation can drive lentiviral replication, we sought to determine the relationship between IFN-gamma-related host immune responses and challenge virus replication in lymphoid tissues of simian-human immunodeficiency virus 89.6 (SHIV89.6)-vaccinated and unvaccinated rhesus macaques 6 months after challenge with simian immunodeficiency virus SIVmac239. Vaccinated-protected monkeys had low tissue viral RNA (vRNA) levels, vaccinated-unprotected animals had moderate tissue vRNA levels, and unvaccinated animals had high tissue vRNA levels. The long-term challenge outcome in vaccinated monkeys was correlated with the relative balance between SIV-specific IFN-gamma T-cell responses and nonspecific IFN-gamma-driven inflammation. Vaccinated-protected monkeys had slightly increased tissue IFN-gamma mRNA levels and a high frequency of IFN-gamma-secreting T cells responding to in vitro SIVgag peptide stimulation; thus, it is likely that they could develop effective anti-SIV cytotoxic T lymphocytes in vivo. In contrast, both high tissue IFN-gamma mRNA levels and strong in vitro SIV-specific IFN-gamma T-cell responses were detected in lymphoid tissues of vaccinated-unprotected monkeys. Unvaccinated monkeys had increased tissue IFN-gamma mRNA levels but weak in vitro anti-SIV IFN-gamma T-cell responses. In addition, in lymphoid tissues of vaccinated-unprotected and unvaccinated monkeys, the increased IFN-gamma mRNA levels were associated with increased Mig/CXCL9, IP-10/CXCL10, and CXCR3 mRNA levels, suggesting that increased Mig/CXCL9 and IP-10/CXCL10 expression resulted in recruitment of CXCR3(+) activated T cells. Thus, IFN-gamma-driven inflammation promotes SIV replication in vaccinated-unprotected and unvaccinated monkeys. Unlike all unvaccinated monkeys, most monkeys vaccinated with SHIV89.6 did not develop IFN-gamma-driven inflammation, but they did develop effective antiviral CD8(+)-T-cell responses.
Figures
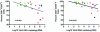


Similar articles
-
High beta-chemokine expression levels in lymphoid tissues of simian/human immunodeficiency virus 89.6-vaccinated rhesus macaques are associated with uncontrolled replication of simian immunodeficiency virus challenge inoculum.J Virol. 2004 Jun;78(12):6399-408. doi: 10.1128/JVI.78.12.6399-6408.2004. J Virol. 2004. PMID: 15163733 Free PMC article.
-
Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses.J Virol. 2003 Mar;77(5):3099-118. doi: 10.1128/jvi.77.5.3099-3118.2003. J Virol. 2003. PMID: 12584336 Free PMC article.
-
Efficacy of a SHIV 89.6 proviral DNA vaccine against mucosal SIVmac239 challenge.Vaccine. 2005 Jul 1;23(31):4036-47. doi: 10.1016/j.vaccine.2005.03.013. Epub 2005 Apr 9. Vaccine. 2005. PMID: 15963361
-
Antiviral CD8+ T cells in the genital tract control viral replication and delay progression to AIDS after vaginal SIV challenge in rhesus macaques immunized with virulence attenuated SHIV 89.6.J Intern Med. 2009 Jan;265(1):67-77. doi: 10.1111/j.1365-2796.2008.02051.x. J Intern Med. 2009. PMID: 19093961 Free PMC article. Review.
-
Immune mechanisms associated with protection from vaginal SIV challenge in rhesus monkeys infected with virulence-attenuated SHIV 89.6.J Med Primatol. 2005 Oct;34(5-6):271-81. doi: 10.1111/j.1600-0684.2005.00125.x. J Med Primatol. 2005. PMID: 16128922 Review.
Cited by
-
Resolution of PMA-induced skin inflammation involves interaction of IFN-γ and ALOX15.Mediators Inflamm. 2013;2013:930124. doi: 10.1155/2013/930124. Epub 2013 Jun 2. Mediators Inflamm. 2013. PMID: 23818745 Free PMC article.
-
Deoxycytidyl-deoxyguanosine oligonucleotide classes A, B, and C induce distinct cytokine gene expression patterns in rhesus monkey peripheral blood mononuclear cells and distinct alpha interferon responses in TLR9-expressing rhesus monkey plasmacytoid dendritic cells.Clin Diagn Lab Immunol. 2005 May;12(5):606-21. doi: 10.1128/CDLI.12.5.606-621.2005. Clin Diagn Lab Immunol. 2005. PMID: 15879022 Free PMC article.
-
Personality and serotonin transporter genotype interact with social context to affect immunity and viral set-point in simian immunodeficiency virus disease.Brain Behav Immun. 2008 Jul;22(5):676-89. doi: 10.1016/j.bbi.2007.05.006. Epub 2007 Aug 23. Brain Behav Immun. 2008. PMID: 17719201 Free PMC article.
-
Ozone and allergen exposure during postnatal development alters the frequency and airway distribution of CD25+ cells in infant rhesus monkeys.Toxicol Appl Pharmacol. 2009 Apr 1;236(1):39-48. doi: 10.1016/j.taap.2008.12.031. Epub 2009 Feb 7. Toxicol Appl Pharmacol. 2009. PMID: 19371618 Free PMC article.
-
Attenuation of V- or C-defective measles viruses: infection control by the inflammatory and interferon responses of rhesus monkeys.J Virol. 2008 Jun;82(11):5359-67. doi: 10.1128/JVI.00169-08. Epub 2008 Apr 2. J Virol. 2008. PMID: 18385234 Free PMC article.
References
-
- Abel, K., M. J. Alegria-Hartman, K. Rothaeusler, M. Marthas, and C. J. Miller. 2002. The relationship between simian immunodeficiency virus RNA levels and the mRNA levels of alpha/beta interferons (IFN-alpha/beta) and IFN-alpha/beta-inducible Mx in lymphoid tissues of rhesus macaques during acute and chronic infection. J. Virol. 76:8433-8445. - PMC - PubMed
-
- Abel, K., M. J. Alegria-Hartman, K. Zanotto, M. B. McChesney, M. L. Marthas, and C. J. Miller. 2001. Anatomic site and immune function correlate with relative cytokine mRNA expression levels in lymphoid tissues of normal rhesus macaques. Cytokine 16:191-204. - PubMed
-
- Abel, K., L. Compton, T. Rourke, D. Montefiori, D. Lu, K. Rothaeusler, L. Fritts, K. Bost, and C. J. Miller. 2003. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J. Virol. 77:3099-3118. - PMC - PubMed
-
- Allen, T. M., T. U. Vogel, D. H. Fuller, B. R. Mothe, S. Steffen, J. E. Boyson, T. Shipley, J. Fuller, T. Hanke, A. Sette, J. D. Altman, B. Moss, A. J. McMichael, and D. I. Watkins. 2000. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J. Immunol. 164:4968-4978. - PubMed
-
- Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8:379-385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

