Chemokine-mediated recruitment of NK cells is a critical host defense mechanism in invasive aspergillosis
- PMID: 14679181
- PMCID: PMC296992
- DOI: 10.1172/JCI18125
Chemokine-mediated recruitment of NK cells is a critical host defense mechanism in invasive aspergillosis
Abstract
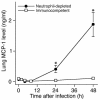
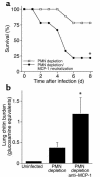
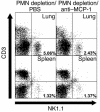
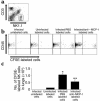
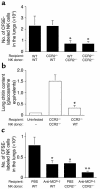
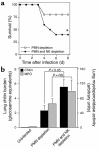
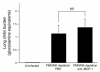
Invasive aspergillosis is a severe pneumonia that is usually fatal despite currently available therapy. The disease disproportionately afflicts immunocompromised patients, indicating the critical importance of the immune status of the host in this infection, but the defense mechanisms against this pathogen remain incompletely understood. In the current study, we hypothesized that the chemokine ligand monocyte chemotactic protein-1, also designated CC chemokine ligand-2 (MCP-1/CCL2) is necessary for effective host defense against invasive aspergillosis in immunocompromised hosts. We found a rapid and marked induction of MCP-1/CCL2 in the lungs of neutropenic mice with invasive aspergillosis. Neutralizing MCP-1/CCL2 resulted in twofold greater mortality and greater than threefold increase in pathogen burden in the lungs. Neutralization of MCP-1/CCL2 also resulted in reduced recruitment of NK cells to the lungs at early time points, but did not affect the number of other leukocyte effector cells in the lungs. Ab-mediated depletion of NK cells similarly resulted in impaired defenses against the infection, resulting in a greater than twofold increase in mortality and impaired clearance of the pathogen from the lungs. These data establish MCP-1/CCL2-mediated recruitment of NK cells to the lungs as a critical early host defense mechanism in invasive aspergillosis and demonstrate NK cells to be an important and previously unrecognized effector cell in this infection.
Figures


Similar articles
-
Macrophage inflammatory protein-1 alpha is a critical mediator of host defense against invasive pulmonary aspergillosis in neutropenic hosts.J Immunol. 2000 Jul 15;165(2):962-8. doi: 10.4049/jimmunol.165.2.962. J Immunol. 2000. PMID: 10878372
-
Early NK cell-derived IFN-{gamma} is essential to host defense in neutropenic invasive aspergillosis.J Immunol. 2009 Apr 1;182(7):4306-12. doi: 10.4049/jimmunol.0803462. J Immunol. 2009. PMID: 19299730 Free PMC article.
-
The role of CC chemokine receptor 6 in host defense in a model of invasive pulmonary aspergillosis.Am J Respir Crit Care Med. 2007 Jun 1;175(11):1165-72. doi: 10.1164/rccm.200602-256OC. Epub 2007 Mar 22. Am J Respir Crit Care Med. 2007. PMID: 17379855 Free PMC article.
-
CCL2 expression is mediated by type I IFN receptor and recruits NK and T cells to the lung during MVA infection.J Leukoc Biol. 2016 Jun;99(6):1057-64. doi: 10.1189/jlb.4MA0815-376RR. Epub 2016 Mar 18. J Leukoc Biol. 2016. PMID: 26992431 Review.
-
Why are natural killer cells important for defense against Aspergillus?Med Mycol. 2019 Apr 1;57(Supplement_2):S206-S210. doi: 10.1093/mmy/myy034. Med Mycol. 2019. PMID: 30816962 Review.
Cited by
-
Hypoxia-inducible factor-dependent induction of myeloid-derived netrin-1 attenuates natural killer cell infiltration during endotoxin-induced lung injury.FASEB J. 2021 Apr;35(4):e21334. doi: 10.1096/fj.202002407R. FASEB J. 2021. PMID: 33715200 Free PMC article.
-
Immunotherapy of invasive fungal infection in hematopoietic stem cell transplant recipients.Front Oncol. 2013 Feb 7;3:17. doi: 10.3389/fonc.2013.00017. eCollection 2013. Front Oncol. 2013. PMID: 23404543 Free PMC article.
-
Lung natural killer cells in mice: phenotype and response to respiratory infection.Immunology. 2012 Sep;137(1):37-47. doi: 10.1111/j.1365-2567.2012.03607.x. Immunology. 2012. PMID: 22612500 Free PMC article.
-
Clinical Features of Fatal Pandemic Influenza A/H1N1 Infection Complicated by Invasive Pulmonary Fungal Infection.Mycopathologia. 2020 Apr;185(2):319-329. doi: 10.1007/s11046-019-00421-z. Epub 2019 Dec 27. Mycopathologia. 2020. PMID: 31883036 Free PMC article.
-
Extra centrosomes induce PIDD1-mediated inflammation and immunosurveillance.EMBO J. 2023 Oct 16;42(20):e113510. doi: 10.15252/embj.2023113510. Epub 2023 Aug 2. EMBO J. 2023. PMID: 37530438 Free PMC article.
References
-
- Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin. Infect. Dis. 2001;32:358–366. - PubMed
-
- McNeil MM, et al. Trends in mortality due to invasive mycotic diseases in the United States, 1980–1997. Clin. Infect. Dis. 2001;33:641–647. - PubMed
-
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. - PubMed
-
- Traynor TR, et al. Differential roles of CC chemokine ligand 2/monocyte chemotactic protein-1 and CCR2 in the development of T1 immunity. J. Immunol. 2002;168:4659–4666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

