Intrapatient alterations in the human immunodeficiency virus type 1 gp120 V1V2 and V3 regions differentially modulate coreceptor usage, virus inhibition by CC/CXC chemokines, soluble CD4, and the b12 and 2G12 monoclonal antibodies
- PMID: 14671134
- PMCID: PMC303404
- DOI: 10.1128/jvi.78.1.524-530.2004
Intrapatient alterations in the human immunodeficiency virus type 1 gp120 V1V2 and V3 regions differentially modulate coreceptor usage, virus inhibition by CC/CXC chemokines, soluble CD4, and the b12 and 2G12 monoclonal antibodies
Abstract
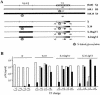
We studied human immunodeficiency virus type 1 (HIV-1) chimeric viruses altering in their gp120 V1V2 and V3 envelope regions to better map which genetic alterations are associated with specific virus phenotypes associated with HIV-1 disease progression. The V1V2 and V3 regions studied were based on viruses isolated from an individual with progressing HIV-1 disease. Higher V3 charges were linked with CXCR4 usage, but only when considered within a specific V1V2 and V3 N-linked glycosylation context. When the virus gained R5X4 dual tropism, irrespective of its V3 charge, it became highly resistant to inhibition by RANTES and highly sensitive to inhibition by SDF-1alpha. R5 viruses with higher positive V3 charges were more sensitive to inhibition by RANTES, while R5X4 dualtropic viruses with higher positive V3 charges were more resistant to inhibition by SDF-1alpha. Loss of the V3 N-linked glycosylation event rendered the virus more resistant to inhibition by SDF-1alpha. The same alterations in the V1V2 and V3 regions influenced the extent to which the viruses were neutralized with soluble CD4, as well as monoclonal antibodies b12 and 2G12, but not monoclonal antibody 2F5. These results further identify a complex set of alterations within the V1V2 and V3 regions of HIV-1 that can be selected in the host via alterations of coreceptor usage, CC/CXC chemokine inhibition, CD4 binding, and antibody neutralization.
Figures

Similar articles
-
Cryptic nature of a conserved, CD4-inducible V3 loop neutralization epitope in the native envelope glycoprotein oligomer of CCR5-restricted, but not CXCR4-using, primary human immunodeficiency virus type 1 strains.J Virol. 2005 Jun;79(11):6957-68. doi: 10.1128/JVI.79.11.6957-6968.2005. J Virol. 2005. PMID: 15890935 Free PMC article.
-
Replacement of the V3 region of gp120 with SDF-1 preserves the infectivity of T-cell line-tropic human immunodeficiency virus type 1.J Virol. 2001 May;75(9):4258-67. doi: 10.1128/JVI.75.9.4258-4267.2001. J Virol. 2001. PMID: 11287575 Free PMC article.
-
Role of V3 independent domains on a dualtropic human immunodeficiency virus type 1 (HIV-1) envelope gp120 in CCR5 coreceptor utilization and viral infectivity.Microbiol Immunol. 2001;45(7):521-30. doi: 10.1111/j.1348-0421.2001.tb02653.x. Microbiol Immunol. 2001. PMID: 11529558
-
Synthetic peptides for study of human immunodeficiency virus infection.Appl Biochem Biotechnol. 2002 Jul-Dec;102-103(1-6):41-7. doi: 10.1385/abab:102-103:1-6:041. Appl Biochem Biotechnol. 2002. PMID: 12396109 Review.
-
HIV-1 infection and chemokine receptor modulation.Curr HIV Res. 2004 Jan;2(1):39-50. doi: 10.2174/1570162043484997. Curr HIV Res. 2004. PMID: 15053339 Review.
Cited by
-
The carbohydrate at asparagine 386 on HIV-1 gp120 is not essential for protein folding and function but is involved in immune evasion.Retrovirology. 2008 Jan 31;5:10. doi: 10.1186/1742-4690-5-10. Retrovirology. 2008. PMID: 18237398 Free PMC article.
-
An oligosaccharide-based HIV-1 2G12 mimotope vaccine induces carbohydrate-specific antibodies that fail to neutralize HIV-1 virions.Proc Natl Acad Sci U S A. 2008 Oct 14;105(41):15684-9. doi: 10.1073/pnas.0807837105. Epub 2008 Oct 6. Proc Natl Acad Sci U S A. 2008. PMID: 18838688 Free PMC article.
-
Varied sensitivity to therapy of HIV-1 strains in CD4+ lymphocyte sub-populations upon ART initiation.AIDS Res Ther. 2010 Dec 6;7:42. doi: 10.1186/1742-6405-7-42. AIDS Res Ther. 2010. PMID: 21134247 Free PMC article.
-
Hierarchical kernel mixture models for the prediction of AIDS disease progression using HIV structural gp120 profiles.BMC Genomics. 2010 Dec 2;11 Suppl 4(Suppl 4):S22. doi: 10.1186/1471-2164-11-S4-S22. BMC Genomics. 2010. PMID: 21143806 Free PMC article.
-
Differential induction of anti-V3 crown antibodies with cradle- and ladle-binding modes in response to HIV-1 envelope vaccination.Vaccine. 2017 Mar 7;35(10):1464-1473. doi: 10.1016/j.vaccine.2016.11.107. Epub 2017 Feb 6. Vaccine. 2017. PMID: 28185743 Free PMC article.
References
-
- Back, N. K. T., L. Smit, J. J. de Jong, W. Keulen, M. Schutten, J. Goudsmit, and M. Tersmette. 1994. An N-glycan within the human immunodeficiency virus type 1 gp120 V3 loop affects virus neutralization. Virology 199:431-438. - PubMed
-
- Berger, E. A. 1997. HIV entry and tropism: the chemokine receptor connection. AIDS 11(Suppl. A):S3-S16. - PubMed
-
- Berger, E. A., R. W. Doms, E. M. Fenyo, B. T. Korber, D. R. Littman, J. P. Moore, Q. J. Sattentau, H. Schuitemaker, J. Sodroski, and R. A. Weiss. 1998. A new classification for HIV-1. Nature 391:240. - PubMed
-
- Bleul, C. C., M. Farzan, H. Choe, C. Parolin, I. Clark-Lewis, J. Sodroski, and T. A. Springer. 1996. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 382:829-833. - PubMed
-
- Bouma, P., M. Leavitt, P. F. Zhang, I. A. Sidorov, D. S. Dimitrov, and G. V. Quinnan, Jr. 2003. Multiple interactions across the surface of the gp120 core structure determine the global neutralization resistance phenotype of human immunodeficiency virus type 1. J. Virol. 77:8061-8071. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

