Sec61p contributes to signal sequence orientation according to the positive-inside rule
- PMID: 14668483
- PMCID: PMC363169
- DOI: 10.1091/mbc.e03-08-0599
Sec61p contributes to signal sequence orientation according to the positive-inside rule
Abstract
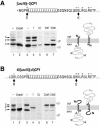
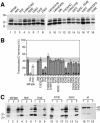
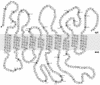
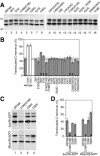
Protein targeting to the endoplasmic reticulum is mediated by signal or signal-anchor sequences. They also play an important role in protein topogenesis, because their orientation in the translocon determines whether their N- or C-terminal sequence is translocated. Signal orientation is primarily determined by charged residues flanking the hydrophobic core, whereby the more positive end is predominantly positioned to the cytoplasmic side of the membrane, a phenomenon known as the "positive-inside rule." We tested the role of conserved charged residues of Sec61p, the major component of the translocon in Saccharomyces cerevisiae, in orienting signals according to their flanking charges by site-directed mutagenesis by using diagnostic model proteins. Mutation of R67, R74, or E382 in Sec61p reduced C-terminal translocation of a signal-anchor protein with a positive N-terminal flanking sequence and increased it for signal-anchor proteins with positive C-terminal sequences. These mutations produced a stronger effect on substrates with greater charge difference across the hydrophobic core of the signal. For some of the substrates, a charge mutation in Sec61p had a similar effect as one in the substrate polypeptides. Although these three residues do not account for the entire charge effect in signal orientation, the results show that Sec61p contributes to the positive-inside rule.
Figures

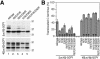
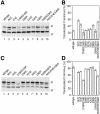
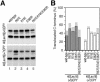
Similar articles
-
Recognition of a subset of signal sequences by Ssh1p, a Sec61p-related protein in the membrane of endoplasmic reticulum of yeast Saccharomyces cerevisiae.Mol Biol Cell. 2002 Jul;13(7):2223-32. doi: 10.1091/mbc.01-10-0518. Mol Biol Cell. 2002. PMID: 12134063 Free PMC article.
-
Interactions between Sec complex and prepro-alpha-factor during posttranslational protein transport into the endoplasmic reticulum.Mol Biol Cell. 2004 Jan;15(1):1-10. doi: 10.1091/mbc.e03-06-0390. Epub 2003 Nov 14. Mol Biol Cell. 2004. PMID: 14617809 Free PMC article.
-
Topogenesis of membrane proteins at the endoplasmic reticulum.Biochemistry. 2004 Oct 12;43(40):12716-22. doi: 10.1021/bi048368m. Biochemistry. 2004. PMID: 15461443 Review.
-
A second trimeric complex containing homologs of the Sec61p complex functions in protein transport across the ER membrane of S. cerevisiae.EMBO J. 1996 Apr 1;15(7):1482-94. EMBO J. 1996. PMID: 8612571 Free PMC article.
-
Heads or tails--what determines the orientation of proteins in the membrane.FEBS Lett. 1995 Aug 1;369(1):76-9. doi: 10.1016/0014-5793(95)00551-j. FEBS Lett. 1995. PMID: 7641889 Review.
Cited by
-
Complexity and Specificity of Sec61-Channelopathies: Human Diseases Affecting Gating of the Sec61 Complex.Cells. 2021 Apr 27;10(5):1036. doi: 10.3390/cells10051036. Cells. 2021. PMID: 33925740 Free PMC article. Review.
-
Positive zip coding in small protein translocation.J Biol Chem. 2018 Feb 9;293(6):1908-1909. doi: 10.1074/jbc.H118.001415. J Biol Chem. 2018. PMID: 29462795 Free PMC article.
-
Anisotropic solvent model of the lipid bilayer. 2. Energetics of insertion of small molecules, peptides, and proteins in membranes.J Chem Inf Model. 2011 Apr 25;51(4):930-46. doi: 10.1021/ci200020k. Epub 2011 Mar 25. J Chem Inf Model. 2011. PMID: 21438606 Free PMC article.
-
Positive charge in the n-region of the signal peptide contributes to efficient post-translational translocation of small secretory preproteins.J Biol Chem. 2018 Feb 9;293(6):1899-1907. doi: 10.1074/jbc.RA117.000922. Epub 2017 Dec 11. J Biol Chem. 2018. PMID: 29229776 Free PMC article.
-
Lipid-protein interactions drive membrane protein topogenesis in accordance with the positive inside rule.J Biol Chem. 2009 Apr 10;284(15):9637-41. doi: 10.1074/jbc.R800081200. Epub 2008 Dec 12. J Biol Chem. 2009. PMID: 19074771 Free PMC article. Review.
References
-
- Beltzer, J.P., Fiedler, K., Fuhrer, C., Geffen, I., Handschin, C., Wessels, H.P., and Spiess, M. (1991). Charged residues are major determinants of the transmembrane orientation of a signal-anchor sequence. J. Biol. Chem. 266, 973-978. - PubMed
-
- Eusebio, A., Friedberg, T., and Spiess, M. (1998). The role of the hydrophobic domain in orienting natural signal sequences within the ER membrane. Exp. Cell Res. 241, 181-185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

