Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation
- PMID: 14662908
- PMCID: PMC2194162
- DOI: 10.1084/jem.20030896
Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation
Abstract
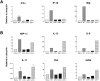
Interleukin (IL) 23 is a heterodimeric cytokine composed of a p19 subunit and the p40 subunit of IL-12. IL-23 affects memory T cell and inflammatory macrophage function through engagement of a novel receptor (IL-23R) on these cells. Recent analysis of the contribution of IL-12 and IL-23 to central nervous system autoimmune inflammation demonstrated that IL-23 rather than IL-12 was the essential cytokine. Using gene-targeted mice lacking only IL-12 (p35-/-) or IL-23 (p19-/-), we show that the specific absence of IL-23 is protective, whereas loss of IL-12 exacerbates collagen-induced arthritis. IL-23 gene-targeted mice did not develop clinical signs of disease and were completely resistant to the development of joint and bone pathology. Resistance correlated with an absence of IL-17-producing CD4+ T cells despite normal induction of collagen-specific, interferon-gamma-producing T helper 1 cells. In contrast, IL-12-deficient p35-/- mice developed more IL-17-producing CD4+ T cells, as well as elevated mRNA expression of proinflammatory tumor necrosis factor, IL-1beta, IL-6, and IL-17 in affected tissues of diseased mice. The data presented here indicate that IL-23 is an essential promoter of end-stage joint autoimmune inflammation, whereas IL-12 paradoxically mediates protection from autoimmune inflammation.
Figures



Similar articles
-
Divergent effects of IL-12 and IL-23 on the production of IL-17 by human T cells.Eur J Immunol. 2006 Mar;36(3):661-70. doi: 10.1002/eji.200535239. Eur J Immunol. 2006. PMID: 16482511
-
Pathophysiology of interleukin-23 in experimental autoimmune encephalomyelitis.Drug News Perspect. 2006 Mar;19(2):77-83. doi: 10.1358/dnp.2006.19.2.977443. Drug News Perspect. 2006. PMID: 16628262 Review.
-
Autoimmune inflammation from the Th17 perspective.Autoimmun Rev. 2007 Jan;6(3):169-75. doi: 10.1016/j.autrev.2006.10.002. Epub 2006 Nov 14. Autoimmun Rev. 2007. PMID: 17289553
-
Prostaglandin E2 exacerbates collagen-induced arthritis in mice through the inflammatory interleukin-23/interleukin-17 axis.Arthritis Rheum. 2007 Aug;56(8):2608-19. doi: 10.1002/art.22794. Arthritis Rheum. 2007. PMID: 17665454
-
Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4.Immunol Rev. 2004 Dec;202:139-56. doi: 10.1111/j.0105-2896.2004.00211.x. Immunol Rev. 2004. PMID: 15546391 Review.
Cited by
-
Immunological cells and functions in Gaucher disease.Crit Rev Oncog. 2013;18(3):197-220. doi: 10.1615/critrevoncog.2013004503. Crit Rev Oncog. 2013. PMID: 23510064 Free PMC article. Review.
-
The IL-23-IL-17 axis in inflammatory arthritis.Nat Rev Rheumatol. 2015 Jul;11(7):415-29. doi: 10.1038/nrrheum.2015.53. Epub 2015 Apr 28. Nat Rev Rheumatol. 2015. PMID: 25907700 Review.
-
To Evaluate the Role and Relevance of Cytokines IL-17, IL-18, IL-23 and TNF-α and Their Correlation with Disease Severity in Chronic Urticaria.Indian Dermatol Online J. 2020 Jan 24;11(4):594-597. doi: 10.4103/idoj.IDOJ_396_19. eCollection 2020 Jul-Aug. Indian Dermatol Online J. 2020. PMID: 32832449 Free PMC article.
-
A Peripheral Blood Signature of Increased Th1 and Myeloid Cells Combined with Serum Inflammatory Mediators Is Associated with Response to Abatacept in Rheumatoid Arthritis Patients.Cells. 2023 Dec 9;12(24):2808. doi: 10.3390/cells12242808. Cells. 2023. PMID: 38132128 Free PMC article.
-
Contribution of the IL-17 Pathway to Psoriasis and Psoriatic Arthritis.Curr Rheumatol Rep. 2015 Aug;17(8):55. doi: 10.1007/s11926-015-0529-9. Curr Rheumatol Rep. 2015. PMID: 26209291 Review.
References
-
- Oppmann, B., R. Lesley, B. Blom, J.C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, et al. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 13:715–725. - PubMed
-
- O'Garra, A., and N. Arai. 2000. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol. 10:542–550. - PubMed
-
- Wu, C., X. Wang, M. Gadina, J.J. O'Shea, D.H. Presky, and J. Magram. 2000. IL-12 receptor beta 2 (IL-12R beta 2)-deficient mice are defective in IL-12-mediated signaling despite the presence of high affinity IL-12 binding sites. J. Immunol. 165:6221–6228. - PubMed
-
- Lankford, C.S., and D.M. Frucht. 2003. A unique role for IL-23 in promoting cellular immunity. J. Leukoc. Biol. 73:49–56. - PubMed
-
- Shtrichman, R., and C.E. Samuel. 2001. The role of gamma interferon in antimicrobial immunity. Curr. Opin. Microbiol. 4:251–259. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

