IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms
- PMID: 14657353
- PMCID: PMC299900
- DOI: 10.1073/pnas.2536517100
IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms
Abstract
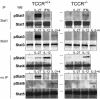
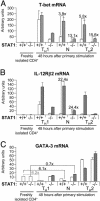
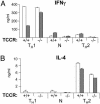
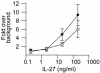
IL-27, a novel heterodimeric cytokine produced by antigen-presenting cells, signals through the T cell cytokine receptor (TCCR)/WSX-1 expressed on naïve CD4+ T cells and natural killer cells. TCCR/WSX-1 deficiency results in delayed T helper type 1 (TH1) development through an unresolved mechanism. We report here that IL-27 stimulation in developing murine T helper cells potently induces the expression of the major TH1-specific transcription factor T-bet and its downstream target IL-12R beta2, independently of IFN gamma. In addition, IL-27 suppresses basal expression of GATA-3, the critical TH2-specific transcription factor that inhibits TH1 development by down-regulating signal transducer and activator of transcription (Stat) 4. IL-27 signaling through TCCR/WSX-1 induces phosphorylation of Stat1, Stat3, Stat4, and Stat5. Stat1 is required for suppression of GATA-3, but T-bet induction by IL-27 can also be mediated through a Stat1-independent pathway. Despite its TH1-like signaling profile, IL-27 is not sufficient to drive the differentiation of CD4+ T cells into IFN gamma-producing cells. Similarly, IL-27 induces T-bet expression in primary natural killer cells, but this does not result in an increase of IFN gamma production or cytotoxic activity. Therefore, although IL-27 is unable to drive IFN gamma production on its own, it plays an important role in the early steps of TH1 commitment by contributing in a paracrine manner to the control of IL-12 responsiveness.
Figures

Similar articles
-
T-bet is a STAT1-induced regulator of IL-12R expression in naïve CD4+ T cells.Nat Immunol. 2002 Jun;3(6):549-57. doi: 10.1038/ni794. Epub 2002 May 13. Nat Immunol. 2002. PMID: 12006974
-
Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment.J Immunol. 2003 May 15;170(10):4886-90. doi: 10.4049/jimmunol.170.10.4886. J Immunol. 2003. PMID: 12734330
-
An indispensable role for STAT1 in IL-27-induced T-bet expression but not proliferation of naive CD4+ T cells.J Immunol. 2004 Sep 15;173(6):3871-7. doi: 10.4049/jimmunol.173.6.3871. J Immunol. 2004. PMID: 15356135
-
T helper differentiation proceeds through Stat1-dependent, Stat4-dependent and Stat4-independent phases.Curr Top Microbiol Immunol. 1999;238:13-26. doi: 10.1007/978-3-662-09709-0_2. Curr Top Microbiol Immunol. 1999. PMID: 10087648 Review.
-
STAT4 and STAT6, their role in cellular and humoral immunity and in diverse human diseases.Int Rev Immunol. 2024;43(6):394-418. doi: 10.1080/08830185.2024.2395274. Epub 2024 Aug 26. Int Rev Immunol. 2024. PMID: 39188021 Review.
Cited by
-
Interleukin-27 in T cell immunity.Int J Mol Sci. 2015 Jan 27;16(2):2851-63. doi: 10.3390/ijms16022851. Int J Mol Sci. 2015. PMID: 25633106 Free PMC article. Review.
-
Pulmonary interleukin-23 gene delivery increases local T-cell immunity and controls growth of Mycobacterium tuberculosis in the lungs.Infect Immun. 2005 Sep;73(9):5782-8. doi: 10.1128/IAI.73.9.5782-5788.2005. Infect Immun. 2005. PMID: 16113296 Free PMC article.
-
Role of cytokines as mediators and regulators of microglial activity in inflammatory demyelination of the CNS.Neuromolecular Med. 2010 Jun;12(2):99-132. doi: 10.1007/s12017-010-8112-z. Epub 2010 Mar 30. Neuromolecular Med. 2010. PMID: 20411441 Review.
-
Transformation of hematopoietic cells and activation of JAK2-V617F by IL-27R, a component of a heterodimeric type I cytokine receptor.Proc Natl Acad Sci U S A. 2007 Nov 20;104(47):18502-7. doi: 10.1073/pnas.0702388104. Epub 2007 Nov 14. Proc Natl Acad Sci U S A. 2007. PMID: 18003935 Free PMC article.
-
Interleukin-27 acts on hepatic stellate cells and induces signal transducer and activator of transcription 1-dependent responses.Cell Commun Signal. 2010 Aug 19;8:19. doi: 10.1186/1478-811X-8-19. Cell Commun Signal. 2010. PMID: 20719000 Free PMC article.
References
-
- Abbas, A. K., Murphy, K. M. & Sher, A. (1996) Nature 383, 787-793. - PubMed
-
- Mosmann, T. R. & Sad, S. (1996) Immunol. Today 17, 138-146. - PubMed
-
- O'Garra, A. (1998) Immunity 8, 275-283. - PubMed
-
- Murphy, K. M. & Reiner, S. L. (2002) Nat. Rev. Immunol. 2, 933-944. - PubMed
-
- Wurster, A. L., Tanaka, T. & Grusby, M. J. (2000) Oncogene 19, 2577-2584. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

