HMG-D and histone H1 alter the local accessibility of nucleosomal DNA
- PMID: 14654683
- PMCID: PMC291865
- DOI: 10.1093/nar/gkg923
HMG-D and histone H1 alter the local accessibility of nucleosomal DNA
Abstract
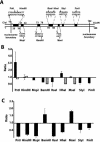
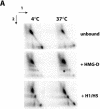
There is evidence that HMGB proteins facilitate, while linker histones inhibit chromatin remodelling, respectively. We have examined the effects of HMG-D and histone H1/H5 on accessibility of nucleosomal DNA. Using the 601.2 nucleosome positioning sequence designed by Widom and colleagues we assembled nucleosomes in vitro and probed DNA accessibility with restriction enzymes in the presence or absence of HMG-D and histone H1/H5. For HMG-D our results show increased digestion at two spatially adjacent sites, the dyad and one terminus of nucleosomal DNA. Elsewhere varying degrees of protection from digestion were observed. The C-terminal acidic tail of HMG-D is essential for this pattern of accessibility. Neither the HMG domain by itself nor in combination with the adjacent basic region is sufficient. Histone H1/H5 binding produces two sites of increased digestion on opposite faces of the nucleosome and decreased digestion at all other sites. Our results provide the first evidence of local changes in the accessibility of nucleosomal DNA upon separate interaction with two linker binding proteins.
Figures

Similar articles
-
A 'one-pot' assay for the accessibility of DNA in a nucleosome core particle.Nucleic Acids Res. 2004 Aug 25;32(15):e122. doi: 10.1093/nar/gnh121. Nucleic Acids Res. 2004. PMID: 15329384 Free PMC article.
-
The footprint of chromosomal proteins HMG-14 and HMG-17 on chromatin subunits.J Mol Biol. 1994 Feb 11;236(1):189-98. doi: 10.1006/jmbi.1994.1128. J Mol Biol. 1994. PMID: 8107104
-
Interaction of maize chromatin-associated HMG proteins with mononucleosomes: role of core and linker histones.Biol Chem. 2003 Jul;384(7):1019-27. doi: 10.1515/BC.2003.114. Biol Chem. 2003. PMID: 12956418
-
Yeast HMO1: Linker Histone Reinvented.Microbiol Mol Biol Rev. 2016 Nov 30;81(1):e00037-16. doi: 10.1128/MMBR.00037-16. Print 2017 Mar. Microbiol Mol Biol Rev. 2016. PMID: 27903656 Free PMC article. Review.
-
Linker histones versus HMG1/2: a struggle for dominance?Bioessays. 1998 Jul;20(7):584-8. doi: 10.1002/(SICI)1521-1878(199807)20:7<584::AID-BIES10>3.0.CO;2-W. Bioessays. 1998. PMID: 9723008 Review.
Cited by
-
Arabidopsis chromatin-associated HMGA and HMGB use different nuclear targeting signals and display highly dynamic localization within the nucleus.Plant Cell. 2006 Nov;18(11):2904-18. doi: 10.1105/tpc.106.047274. Epub 2006 Nov 17. Plant Cell. 2006. PMID: 17114349 Free PMC article.
-
Structural insights into the mechanism of negative regulation of single-box high mobility group proteins by the acidic tail domain.J Biol Chem. 2014 Oct 24;289(43):29817-26. doi: 10.1074/jbc.M114.591115. Epub 2014 Sep 4. J Biol Chem. 2014. PMID: 25190813 Free PMC article.
-
High-mobility-group box nuclear factors of Plasmodium falciparum.Eukaryot Cell. 2006 Apr;5(4):672-82. doi: 10.1128/EC.5.4.672-682.2006. Eukaryot Cell. 2006. PMID: 16607015 Free PMC article.
-
A translational signature for nucleosome positioning in vivo.Nucleic Acids Res. 2009 Sep;37(16):5309-21. doi: 10.1093/nar/gkp574. Epub 2009 Jul 13. Nucleic Acids Res. 2009. PMID: 19596807 Free PMC article.
-
Genome-Wide Profiling of PARP1 Reveals an Interplay with Gene Regulatory Regions and DNA Methylation.PLoS One. 2015 Aug 25;10(8):e0135410. doi: 10.1371/journal.pone.0135410. eCollection 2015. PLoS One. 2015. PMID: 26305327 Free PMC article.
References
-
- Luger K., Mader,A.W., Richmond,R.K., Sargent,D.F. and Richmond,T.J. (1997) Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature, 389, 251–260. - PubMed
-
- Anderson J.D. and Widom,J. (2000) Sequence and position-dependence of the equilibrium accessibility of nucleosomal DNA target sites. J. Mol. Biol., 296, 979–987. - PubMed
-
- Polach K.J. and Widom,J. (1995) Mechanism of protein access to specific DNA sequences in chromatin: a dynamic equilibrium model for gene regulation. J. Mol. Biol., 254, 130–149. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

