GacA regulates symbiotic colonization traits of Vibrio fischeri and facilitates a beneficial association with an animal host
- PMID: 14645281
- PMCID: PMC296250
- DOI: 10.1128/JB.185.24.7202-7212.2003
GacA regulates symbiotic colonization traits of Vibrio fischeri and facilitates a beneficial association with an animal host
Abstract
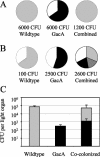
The GacS/GacA two-component system regulates the expression of bacterial traits during host association. Although the importance of GacS/GacA as a regulator of virulence is well established, its role in benign associations is not clear, as mutations in either the gacS or gacA gene have little impact on the success of colonization in nonpathogenic associations studied thus far. Using as a model the symbiotic association of the bioluminescent marine bacterium Vibrio fischeri with its animal host, the Hawaiian bobtail squid, Euprymna scolopes, we investigated the role of GacA in this beneficial animal-microbe interaction. When grown in culture, gacA mutants were defective in several traits important for symbiosis, including luminescence, growth in defined media, growth yield, siderophore activity, and motility. However, gacA mutants were not deficient in production of acylated homoserine lactone signals or catalase activity. The ability of the gacA mutants to initiate squid colonization was impaired but not abolished, and they reached lower-than-wild-type population densities within the host light organ. In contrast to their dark phenotype in culture, gacA mutants that reached population densities above the luminescence detection limit had normal levels of luminescence per bacterial cell in squid light organs, indicating that GacA is not required for light production within the host. The gacA mutants were impaired at competitive colonization and could only successfully cocolonize squid light organs when present in the seawater at higher inoculum densities than wild-type bacteria. Although severely impaired during colonization initiation, gacA mutants were not displaced by the wild-type strain in light organs that were colonized with both strains. This study establishes the role of GacA as a regulator of a beneficial animal-microbe association and indicates that GacA regulates utilization of growth substrates as well as other colonization traits.
Figures


Similar articles
-
The Vibrio fischeri quorum-sensing systems ain and lux sequentially induce luminescence gene expression and are important for persistence in the squid host.Mol Microbiol. 2003 Oct;50(1):319-31. doi: 10.1046/j.1365-2958.2003.t01-1-03585.x. Mol Microbiol. 2003. PMID: 14507383
-
Competition between Vibrio fischeri strains during initiation and maintenance of a light organ symbiosis.J Bacteriol. 1994 Apr;176(7):1985-91. doi: 10.1128/jb.176.7.1985-1991.1994. J Bacteriol. 1994. PMID: 8144466 Free PMC article.
-
Contribution of pilA to competitive colonization of the squid Euprymna scolopes by Vibrio fischeri.Appl Environ Microbiol. 2003 Feb;69(2):820-6. doi: 10.1128/AEM.69.2.820-826.2003. Appl Environ Microbiol. 2003. PMID: 12571000 Free PMC article.
-
Lessons from a cooperative, bacterial-animal association: the Vibrio fischeri-Euprymna scolopes light organ symbiosis.Annu Rev Microbiol. 1996;50:591-624. doi: 10.1146/annurev.micro.50.1.591. Annu Rev Microbiol. 1996. PMID: 8905092 Review.
-
Quorum sensing in the squid-Vibrio symbiosis.Int J Mol Sci. 2013 Aug 7;14(8):16386-401. doi: 10.3390/ijms140816386. Int J Mol Sci. 2013. PMID: 23965960 Free PMC article. Review.
Cited by
-
Two-component response regulators of Vibrio fischeri: identification, mutagenesis, and characterization.J Bacteriol. 2007 Aug;189(16):5825-38. doi: 10.1128/JB.00242-07. Epub 2007 Jun 22. J Bacteriol. 2007. PMID: 17586650 Free PMC article.
-
Common trends in mutualism revealed by model associations between invertebrates and bacteria.FEMS Microbiol Rev. 2010 Jan;34(1):41-58. doi: 10.1111/j.1574-6976.2009.00193.x. FEMS Microbiol Rev. 2010. PMID: 19909347 Free PMC article. Review.
-
Characterizing the host and symbiont proteomes in the association between the Bobtail squid, Euprymna scolopes, and the bacterium, Vibrio fischeri.PLoS One. 2011;6(10):e25649. doi: 10.1371/journal.pone.0025649. Epub 2011 Oct 5. PLoS One. 2011. PMID: 21998678 Free PMC article.
-
Two Polyketides Intertwined in Complex Regulation: Posttranscriptional CsrA-Mediated Control of Colibactin and Yersiniabactin Synthesis in Escherichia coli.mBio. 2021 Feb 22;13(1):e0381421. doi: 10.1128/mbio.03814-21. Epub 2022 Feb 1. mBio. 2021. PMID: 35100864 Free PMC article.
-
Prediction of CsrA-regulating small RNAs in bacteria and their experimental verification in Vibrio fischeri.Nucleic Acids Res. 2006 Jul 5;34(11):3361-9. doi: 10.1093/nar/gkl439. Print 2006. Nucleic Acids Res. 2006. PMID: 16822857 Free PMC article.
References
-
- Ausubel, F., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1990. Current protocols in molecular biology. Wiley and Sons, Inc., New York, N.Y.
-
- Beers, R. F., Jr., and I. W. Sizer. 1952. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 195:133-140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

