Enhanced spontaneous activity of the mu opioid receptor by cysteine mutations: characterization of a tool for inverse agonist screening
- PMID: 14641935
- PMCID: PMC317294
- DOI: 10.1186/1471-2210-3-14
Enhanced spontaneous activity of the mu opioid receptor by cysteine mutations: characterization of a tool for inverse agonist screening
Abstract
Background: The concept of spontaneous- or constitutive-activity has become widely accepted and verified for numerous G protein-coupled receptors and this ligand-independent activity is also acknowledged to play a role in some pathologies. Constitutive activity has been reported for the mu opioid receptor. In some cases the increase in receptor basal activity was induced by chronic morphine administration suggesting that constitutive activity may contribute to the development of drug tolerance and dependence. Constitutively active mutants represent excellent tools for gathering information about the mechanisms of receptor activation and the possible physiological relevance of spontaneous receptor activity. The high basal level of activity of these mutants also allows for easier identification of inverse agonists, defined as ligands able to suppress spontaneous receptor activity, and leads to a better comprehension of their modulatory effects as well as possible in vivo use.
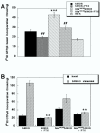
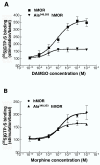
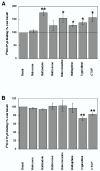
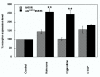
Results: Cysteines 348 and 353 of the human mu opioid receptor (hMOR) were mutated into alanines and Ala348,353 hMOR was stably expressed in HEK 293 cells. [35S] GTPgammaS binding experiments revealed that Ala348,353 hMOR basal activity was significantly higher when compared to hMOR, suggesting that the mutant receptor is constitutively active. [35S] GTPgammaS binding was decreased by cyprodime or CTOP indicating that both ligands have inverse agonist properties. All tested agonists exhibited binding affinities higher for Ala348,353 hMOR than for hMOR, with the exception of endogenous opioid peptides. Antagonist affinity remained virtually unchanged except for CTOP and cyprodime that bound the double mutant with higher affinities. The agonists DAMGO and morphine showed enhanced potency for the Ala348,353 hMOR receptor in [35S] GTPgammaS experiments. Finally, pretreatment with the antagonists naloxone, cyprodime or CTOP significantly increased Ala348,353 hMOR expression.
Conclusion: Taken together our data indicate that the double C348/353A mutation results in a constitutively active conformation of hMOR that is still activated by agonists. This is the first report of a stable CAM of hMOR with the potential to screen for inverse agonists.
Figures
Similar articles
-
Functional role of a conserved motif in TM6 of the rat mu opioid receptor: constitutively active and inactive receptors result from substitutions of Thr6.34(279) with Lys and Asp.Biochemistry. 2001 Nov 13;40(45):13501-9. doi: 10.1021/bi010917q. Biochemistry. 2001. PMID: 11695897
-
Chronic agonist treatment converts antagonists into inverse agonists at delta-opioid receptors.J Pharmacol Exp Ther. 2002 Sep;302(3):1070-9. doi: 10.1124/jpet.102.035964. J Pharmacol Exp Ther. 2002. PMID: 12183665
-
Stimulation of guanosine-5'-o-(3-[35S]thio)triphosphate binding in digitonin-permeabilized C6 rat glioma cells: evidence for an organized association of mu-opioid receptors and G protein.J Pharmacol Exp Ther. 2001 Jul;298(1):116-21. J Pharmacol Exp Ther. 2001. PMID: 11408532
-
Agonist induced constitutive receptor activation as a novel regulatory mechanism. Mu receptor regulation.Adv Exp Med Biol. 1995;373:85-90. doi: 10.1007/978-1-4615-1951-5_12. Adv Exp Med Biol. 1995. PMID: 7668164 Review.
-
Inverse agonism at heptahelical receptors: concept, experimental approach and therapeutic potential.Fundam Clin Pharmacol. 2000 Mar-Apr;14(2):73-87. doi: 10.1111/j.1472-8206.2000.tb00395.x. Fundam Clin Pharmacol. 2000. PMID: 10796054 Review.
Cited by
-
Shared mechanisms for opioid tolerance and a transition to chronic pain.J Neurosci. 2010 Mar 31;30(13):4660-6. doi: 10.1523/JNEUROSCI.5530-09.2010. J Neurosci. 2010. PMID: 20357116 Free PMC article.
-
Post-activation-mediated changes in opioid receptors detected by N-terminal antibodies.J Biol Chem. 2008 Apr 18;283(16):10735-44. doi: 10.1074/jbc.M709454200. Epub 2008 Feb 6. J Biol Chem. 2008. PMID: 18256033 Free PMC article.
-
Preservation of eumelanin hair pigmentation in proopiomelanocortin-deficient mice on a nonagouti (a/a) genetic background.Endocrinology. 2005 Mar;146(3):1245-53. doi: 10.1210/en.2004-0733. Epub 2004 Nov 24. Endocrinology. 2005. PMID: 15564334 Free PMC article.
-
Neutral antagonist activity of naltrexone and 6beta-naltrexol in naïve and opioid-dependent C6 cells expressing a mu-opioid receptor.Br J Pharmacol. 2009 Apr;156(7):1044-53. doi: 10.1111/j.1476-5381.2008.00035.x. Epub 2009 Feb 13. Br J Pharmacol. 2009. PMID: 19220294 Free PMC article.
-
The role of N53Q mutation on the rat mu-opioid receptor function.J Biomol Tech. 2010 Jul;21(2):92-6. J Biomol Tech. 2010. PMID: 20592872 Free PMC article.
References
-
- Massotte D, Kieffer BL. A molecular basis for opioid action. Essays Biochem. 1998;33:65–77. - PubMed
-
- Brys R, Josson K, Castelli MP, Jurzak M, Lijnen P, Gommeren W, Leysen JE. Reconstitution of the human 5-HT1D receptor-G-protein coupling: evidence for constitutive activity and multiple receptor conformations. Mol Pharmacol. 2000;57:1132–1141. - PubMed
-
- Pauwels PJ, Wurch T. Amino acid domains involved in constitutive activation of G protein-coupled receptors. Mol Neurobiol. 1998;17:109–135. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

