Microscopic analysis of the cellular events during scatter factor/hepatocyte growth factor-induced epithelial tubulogenesis
- PMID: 14635802
- PMCID: PMC1571189
- DOI: 10.1046/j.1469-7580.2003.00238.x
Microscopic analysis of the cellular events during scatter factor/hepatocyte growth factor-induced epithelial tubulogenesis
Abstract
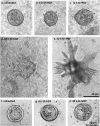
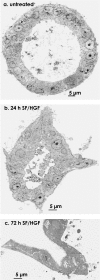
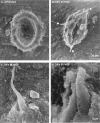
Scatter factor/hepatocyte growth factor (SF/HGF), a large multifunctional polypeptide growth and motility factor, is known to play important roles during embryonic development, adult tissue growth and repair. In an established three-dimensional type I collagen model, SF/HGF induces Madin-Darby canine kidney (MDCK) epithelial cysts to form long, branching tubules (tubulogenesis). In addition, the composition of the surrounding extracellular matrix (ECM) has been shown to modulate SF/HGF-induced morphogenesis, where tubulogenesis was completely abrogated in Matrigel basement membrane. Many cellular events that occur during SF/HGF-mediated remodelling, and its modulation by the ECM, remain unclear. We have investigated these mechanisms through microscopic examination of the time-course of SF/HGF-induced responses in MDCK cysts cultured in type I collagen or Matrigel. We found that early responses to SF/HGF were matrix-independent. Changes included increased paracellular spacing between normally closely apposed lateral membranes, and the formation of filopodial processes, indicating a partial motile response. Cell-cell contact was maintained, with the persistence of cell junctions. Therefore, while one or a number of ECM components are preventing SF/HGF-primed cells from undergoing an invasive and/or migratory programme, non-permissive matrices are not preventing SF/HGF signalling to the cell. Later matrix-dependent responses, which occurred in type I collagen but not Matrigel, included the formation of basal protrusions that comprise two or more neighbouring cells, which extend to form nascent tubules. Modified polarity of cells comprising the basal protrusions was evident, with a marker for the apical membrane being found in the same region as adherens junctions and desmosomes, typically localized at lateral membranes. We propose a model for SF/HGF-induced tubulogenesis in which tubules form from basal protrusions of adjacent cells. This mechanism of in vitro tubule formation has many similarities to reported in vivo epithelial tubulogenesis.
Figures








Similar articles
-
HGF-induced tubulogenesis and branching of epithelial cells is modulated by extracellular matrix and TGF-beta.Dev Biol. 1993 Dec;160(2):293-302. doi: 10.1006/dbio.1993.1308. Dev Biol. 1993. PMID: 8253265
-
Inhibition of junction assembly in cultured epithelial cells by hepatocyte growth factor/scatter factor is concomitant with increased stability and altered phosphorylation of the soluble junctional molecules.Cell Growth Differ. 1997 Apr;8(4):451-62. Cell Growth Differ. 1997. PMID: 9101091
-
Transforming growth factor-beta selectively inhibits branching morphogenesis but not tubulogenesis.Am J Physiol. 1997 Jan;272(1 Pt 2):F139-46. doi: 10.1152/ajprenal.1997.272.1.F139. Am J Physiol. 1997. PMID: 9039060
-
HGF/SF-met signaling in the control of branching morphogenesis and invasion.J Cell Biochem. 2003 Feb 1;88(2):408-17. doi: 10.1002/jcb.10358. J Cell Biochem. 2003. PMID: 12520544 Review.
-
How to make tubes: signaling by the Met receptor tyrosine kinase.Trends Cell Biol. 2003 Jun;13(6):328-35. doi: 10.1016/s0962-8924(03)00104-1. Trends Cell Biol. 2003. PMID: 12791299 Review.
Cited by
-
Critical role of TRPC6 channels in the development of human renal cell carcinoma.Mol Biol Rep. 2013 Aug;40(8):5115-22. doi: 10.1007/s11033-013-2613-4. Epub 2013 May 23. Mol Biol Rep. 2013. PMID: 23700295
-
Pak1 regulates branching morphogenesis in 3D MDCK cell culture by a PIX and beta1-integrin-dependent mechanism.Am J Physiol Cell Physiol. 2010 Jul;299(1):C21-32. doi: 10.1152/ajpcell.00543.2009. Epub 2010 Mar 24. Am J Physiol Cell Physiol. 2010. PMID: 20457839 Free PMC article.
-
A potential role of progestin-induced laminin-5/α6-integrin signaling in the formation of side branches in the mammary gland.Endocrinology. 2012 Oct;153(10):4990-5001. doi: 10.1210/en.2012-1518. Epub 2012 Aug 21. Endocrinology. 2012. PMID: 22910029 Free PMC article.
-
Collective epithelial cell invasion overcomes mechanical barriers of collagenous extracellular matrix by a narrow tube-like geometry and MMP14-dependent local softening.Integr Biol (Camb). 2011 Dec;3(12):1153-66. doi: 10.1039/c1ib00073j. Epub 2011 Oct 13. Integr Biol (Camb). 2011. PMID: 21993836 Free PMC article.
-
Regulation of PCNA and cyclin D1 expression and epithelial morphogenesis by the ZO-1-regulated transcription factor ZONAB/DbpA.Mol Cell Biol. 2006 Mar;26(6):2387-98. doi: 10.1128/MCB.26.6.2387-2398.2006. Mol Cell Biol. 2006. PMID: 16508013 Free PMC article.
References
-
- Affolter M, Bellusci S, Itoh N, Shilo B, Thiery JP, Werb Z. Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev. cell. 2003;4:11–18. 10.1046/j.1469-7580.2003.00238.x. - DOI - PubMed
-
- Alford D, Baeckstrom D, Geyp M, Pitha P, Taylor-Papadimitrriou J. Integrin–matrix interactions affect the form of the structures developing from human mammary epithelial cells in collagen or fibrin gels. J. Cell Sci. 1998;111:521–532. 10.1046/j.1469-7580.2003.00238.x. - DOI - PubMed
-
- Balkovetz DF. Evidence that hepatocyte growth factor abrogates contact inhibition of mitosis in Madin-Darby canine kidney cell monolayers. Life Sci. 1999;64:1393–1401. 10.1046/j.1469-7580.2003.00238.x. - DOI - PubMed
-
- Berdichevsky F, Alford D, D’Souza B, Taylor-Papadimitriou J. Branching morphogenesis of human mammary epithelial cells in collagen gels. J. Cell Sci. 1994;107:3557–3568. 10.1046/j.1469-7580.2003.00238.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

