Divergent functions of neuronal Rab11b in Ca2+-regulated versus constitutive exocytosis
- PMID: 14627637
- PMCID: PMC6740915
- DOI: 10.1523/JNEUROSCI.23-33-10531.2003
Divergent functions of neuronal Rab11b in Ca2+-regulated versus constitutive exocytosis
Abstract
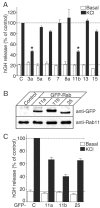
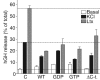
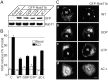
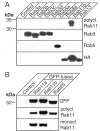
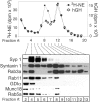
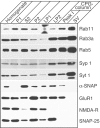
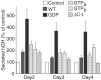
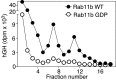
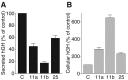
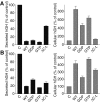
Using PC12 cells that express transfected human growth hormone (hGH) as a secreted reporter protein, we have searched for Rab proteins that function in exocytosis. Among the Rab proteins tested, we found that besides the previously described Rab3 proteins, only members of the Rab11 family (Rab11a, 11b, and 25) impaired Ca2+-induced exocytosis. Rab11b, which is enriched in brain, had the strongest effect. Consistent with a role in exocytosis, Rab11 and Rab3 proteins were colocalized with other vesicle proteins on secretory vesicles in PC12 cells and on mature synaptic vesicles in brain. Rab11b mutants that fix Rab11b in the GTP- or GDP-bound state both effectively inhibited Ca2+-induced exocytosis but seemed to act by distinct mechanisms: whereas GDP-bound Rab11b greatly stimulated constitutive secretion of hGH and depleted hGH stores in secretory vesicles, GTP-bound Rab11b only had a moderate effect on constitutive secretion and no effect on vesicular hGH stores. These results suggest that, consistent with a GTP-dependent regulation of Rab function, GDP-bound Rab11b indirectly inhibits Ca2+-triggered exocytosis by causing the loss of hGH from the PC12 cells, whereas GTP-bound Rab11b directly impairs Ca2+-triggered exocytosis. In contrast to neuroendocrine PC12 cells in which GTP- and GDP-bound Rab11b inhibited Ca2+-induced, but not constitutive, exocytosis, in non-neuronal cells GTP- and GDP-bound Rab11b inhibited constitutive exocytosis and caused an accumulation of cellular hGH. Viewed together, our data suggest that, in addition to other functions, Rab11 has a specific role in neuronal and neuroendocrine but not in non-neuronal cells as a GTP-dependent switch between regulated and constitutive secretory pathways.
Figures
Similar articles
-
The Rab-binding protein Noc2 is associated with insulin-containing secretory granules and is essential for pancreatic beta-cell exocytosis.Mol Endocrinol. 2004 Jan;18(1):117-26. doi: 10.1210/me.2003-0300. Epub 2003 Oct 30. Mol Endocrinol. 2004. PMID: 14593078
-
Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion.Nature. 1997 Aug 7;388(6642):593-8. doi: 10.1038/41580. Nature. 1997. PMID: 9252191
-
Quantitative analysis of synaptic vesicle Rabs uncovers distinct yet overlapping roles for Rab3a and Rab27b in Ca2+-triggered exocytosis.J Neurosci. 2010 Oct 6;30(40):13441-53. doi: 10.1523/JNEUROSCI.0907-10.2010. J Neurosci. 2010. PMID: 20926670 Free PMC article.
-
Regulation of secretory vesicle traffic by Rab small GTPases.Cell Mol Life Sci. 2008 Sep;65(18):2801-13. doi: 10.1007/s00018-008-8351-4. Cell Mol Life Sci. 2008. PMID: 18726178 Free PMC article. Review.
-
GTP- and GDP-Dependent Rab27a Effectors in Pancreatic Beta-Cells.Biol Pharm Bull. 2015;38(5):663-8. doi: 10.1248/bpb.b14-00886. Biol Pharm Bull. 2015. PMID: 25947911 Review.
Cited by
-
The membrane trafficking and functionality of the K+-Cl- co-transporter KCC2 is regulated by TGF-β2.J Cell Sci. 2016 Sep 15;129(18):3485-98. doi: 10.1242/jcs.189860. Epub 2016 Aug 5. J Cell Sci. 2016. PMID: 27505893 Free PMC article.
-
Unbiased Thiol-Labeling and Top-Down Proteomic Analyses Implicate Multiple Proteins in the Late Steps of Regulated Secretion.Proteomes. 2019 Sep 27;7(4):34. doi: 10.3390/proteomes7040034. Proteomes. 2019. PMID: 31569819 Free PMC article.
-
Presynaptic calcium channel localization and calcium-dependent synaptic vesicle exocytosis regulated by the Fuseless protein.J Neurosci. 2008 Apr 2;28(14):3668-82. doi: 10.1523/JNEUROSCI.5553-07.2008. J Neurosci. 2008. PMID: 18385325 Free PMC article.
-
Rab11a-dependent exocytosis of discoidal/fusiform vesicles in bladder umbrella cells.Proc Natl Acad Sci U S A. 2008 Oct 14;105(41):15773-8. doi: 10.1073/pnas.0805636105. Epub 2008 Oct 8. Proc Natl Acad Sci U S A. 2008. PMID: 18843107 Free PMC article.
-
Souffle/Spastizin controls secretory vesicle maturation during zebrafish oogenesis.PLoS Genet. 2014 Jun 26;10(6):e1004449. doi: 10.1371/journal.pgen.1004449. eCollection 2014 Jun. PLoS Genet. 2014. PMID: 24967841 Free PMC article.
References
-
- Calhoun BC, Lapierre LA, Chew CS, Goldenring JR ( 1998) Rab11a redistributes to apical secretory canaliculus during stimulation of gastric parietal cells. Am J Physiol 275: C163-C170. - PubMed
-
- Castillo PE, Janz R, Südhof TC, Tzounopoulos T, Malenka RC, Nicoll RA ( 1997) Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature 388: 590-593. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
