Nuclear translocation of 3'-phosphoinositide-dependent protein kinase 1 (PDK-1): a potential regulatory mechanism for PDK-1 function
- PMID: 14623982
- PMCID: PMC283536
- DOI: 10.1073/pnas.2335486100
Nuclear translocation of 3'-phosphoinositide-dependent protein kinase 1 (PDK-1): a potential regulatory mechanism for PDK-1 function
Abstract
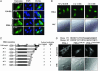
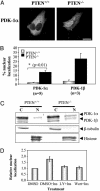
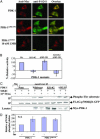
3'-Phosphoinositide-dependent protein kinase 1 (PDK-1) phosphorylates and activates members of the AGC protein kinase family and plays an important role in the regulation of cell survival, differentiation, and proliferation. However, how PDK-1 is regulated in cells remains elusive. In this study, we demonstrated that PDK-1 can shuttle between the cytoplasm and nucleus. Treatment of cells with leptomycin B, a nuclear export inhibitor, results in a nuclear accumulation of PDK-1. PDK-1 nuclear localization is increased by insulin, and this process is inhibited by pretreatment of cells with phosphatidylinositol 3-kinase (PI3-kinase) inhibitors. Consistent with the idea that PDK-1 nuclear translocation is regulated by the PI3-kinase signaling pathway, PDK-1 nuclear localization is increased in cells deficient of PTEN (phosphatase and tensin homologue deleted on chromosome 10). Deletion mapping and mutagenesis studies unveiled that presence of a functional nuclear export signal (NES) in mouse PDK-1 located at amino acid residues 382 to 391. Overexpression of constitutively nuclear PDK-1, which retained autophosphorylation at Ser-244 in the activation loop in cells and its kinase activity in vitro, led to increased phosphorylation of the predominantly nuclear PDK-1 substrate p70 S6KbetaI. However, the ability of constitutively nuclear PDK-1 to induce anchorage-independent growth and to protect against UV-induced apoptosis is greatly diminished compared with the wild-type enzyme. Taken together, these findings suggest that nuclear translocation may be a mechanism to sequestrate PDK-1 from activation of the cytosolic signaling pathways and that this process may play an important role in regulating PDK-1-mediated cell signaling and function.
Figures

Similar articles
-
Loss of PTEN expression does not contribute to PDK-1 activity and PKC activation-loop phosphorylation in Jurkat leukaemic T cells.Cell Signal. 2007 Dec;19(12):2444-57. doi: 10.1016/j.cellsig.2007.07.020. Epub 2007 Aug 3. Cell Signal. 2007. PMID: 17826953
-
Extracellular signal-regulated kinase 2 (ERK-2) mediated phosphorylation regulates nucleo-cytoplasmic shuttling and cell growth control of Ras-associated tumor suppressor protein, RASSF2.Exp Cell Res. 2009 Oct 1;315(16):2775-90. doi: 10.1016/j.yexcr.2009.06.013. Epub 2009 Jun 23. Exp Cell Res. 2009. PMID: 19555684
-
The major target of the endogenously generated reactive oxygen species in response to insulin stimulation is phosphatase and tensin homolog and not phosphoinositide-3 kinase (PI-3 kinase) in the PI-3 kinase/Akt pathway.Mol Biol Cell. 2005 Jan;16(1):348-57. doi: 10.1091/mbc.e04-05-0369. Epub 2004 Nov 10. Mol Biol Cell. 2005. PMID: 15537704 Free PMC article.
-
3'-phosphoinositide-dependent kinase-1 (PDK-1) in PI 3-kinase signaling.Front Biosci. 2002 Apr 1;7:d886-902. doi: 10.2741/storz. Front Biosci. 2002. PMID: 11897568 Review.
-
Pten signaling in gliomas.Neuro Oncol. 2002 Jul;4(3):196-211. Neuro Oncol. 2002. PMID: 12084351 Free PMC article. Review.
Cited by
-
Adiponectin activates AMP-activated protein kinase in muscle cells via APPL1/LKB1-dependent and phospholipase C/Ca2+/Ca2+/calmodulin-dependent protein kinase kinase-dependent pathways.J Biol Chem. 2009 Aug 14;284(33):22426-22435. doi: 10.1074/jbc.M109.028357. Epub 2009 Jun 11. J Biol Chem. 2009. PMID: 19520843 Free PMC article.
-
Phosphoinositide-dependent phosphorylation of PDK1 regulates nuclear translocation.Mol Cell Biol. 2005 Mar;25(6):2347-63. doi: 10.1128/MCB.25.6.2347-2363.2005. Mol Cell Biol. 2005. PMID: 15743829 Free PMC article.
-
Activation of the essential kinase PDK1 by phosphoinositide-driven trans-autophosphorylation.Nat Commun. 2022 Apr 6;13(1):1874. doi: 10.1038/s41467-022-29368-4. Nat Commun. 2022. PMID: 35387990 Free PMC article.
-
The nuts and bolts of AGC protein kinases.Nat Rev Mol Cell Biol. 2010 Jan;11(1):9-22. doi: 10.1038/nrm2822. Nat Rev Mol Cell Biol. 2010. PMID: 20027184 Review.
-
Where is mTOR and what is it doing there?J Cell Biol. 2013 Nov 25;203(4):563-74. doi: 10.1083/jcb.201306041. J Cell Biol. 2013. PMID: 24385483 Free PMC article. Review.
References
-
- Alessi, D. R., James, S. R., Downes, C. P., Holmes, A. B., Gaffney, P. R., Reese, C. B. & Cohen, P. (1997) Curr. Biol. 7, 261–269. - PubMed
-
- Alessi, D. R., Kozlowski, M. T., Weng, Q. P., Morrice, N. & Avruch, J. (1998) Curr. Biol. 8, 69–81. - PubMed
-
- Pullen, N., Dennis, P. B., Andjelkovic, M., Dufner, A., Kozma, S. C., Hemmings, B. A. & Thomas, G. (1998) Science 279, 707–710. - PubMed
-
- Minami, T., Hara, K., Oshiro, N., Ueoku, S., Yoshino, K., Tokunaga, C., Shirai, Y., Saito, N., Gout, I. & Yonezawa, K. (2001) Genes Cells 6, 1003–1015. - PubMed
-
- Lee-Fruman, K. K., Kuo, C. J., Lippincott, J., Terada, N. & Blenis, J. (1999) Oncogene 18, 5108–5114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

