The murine G+C-rich promoter binding protein mGPBP is required for promoter-specific transcription
- PMID: 14612417
- PMCID: PMC262660
- DOI: 10.1128/MCB.23.23.8773-8785.2003
The murine G+C-rich promoter binding protein mGPBP is required for promoter-specific transcription
Abstract
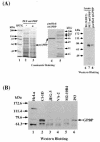
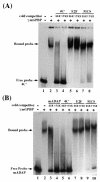
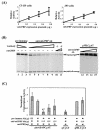
The archetypal TATA-box deficient G+C-rich promoter of the murine adenosine deaminase gene (Ada) requires a 48-bp minimal self-sufficient promoter element (MSPE) for function. This MSPE was used to isolate a novel full-length cDNA clone that encodes a 66-kDa murine G+C-rich promoter binding protein (mGPBP). The mGPBP mRNAs are ubiquitously expressed as either 3.0- or 3.5-kb forms differing in 3' polyadenylation site usage. Purified recombinant mGPBP, in the absence of any other mammalian cofactors, binds specifically to both the murine Ada gene promoter's MSPE and the nonhomologous human Topo IIalpha gene's G+C-rich promoter. In situ binding assays, immunoprecipitation, and Western blot analyses demonstrated that mGPBP is a nuclear factor that can form complexes with TATA-binding protein, TFIIB, TFIIF, RNA polymerase II, and P300/CBP both in vitro and in intact cells. In cotransfection assays, increased mGPBP expression transactivated the murine Ada gene's promoter. Sequestering of GPBP present in HeLa cell nuclear extract by immunoabsorption completely and reversibly suppressed extract-dependent in vitro transcription from the murine Ada gene's G+C-rich promoter. However, transcription from the human Topo IIalpha gene's TATA box-containing G+C-rich promoter was only partially suppressed and the adenovirus major late gene's classical TATA box-dependent promoter is totally unaffected under identical assay conditions. These results implicate GPBP as a requisite G+C-rich promoter-specific transcription factor and provide a mechanistic basis for distinguishing transcription initiated at a TATA box-deficient G+C-rich promoter from that initiated at a TATA box-dependent promoter.
Figures

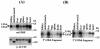



Similar articles
-
The minimal self-sufficient element in a murine G+C-rich promoter is a large element with imperfect dyad symmetry.Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11865-9. doi: 10.1073/pnas.90.24.11865. Proc Natl Acad Sci U S A. 1993. PMID: 8265639 Free PMC article.
-
The murine adenosine deaminase promoter requires an atypical TATA box which binds transcription factor IID and transcriptional activity is stimulated by multiple upstream Sp1 binding sites.J Biol Chem. 1991 Nov 15;266(32):21765-72. J Biol Chem. 1991. PMID: 1939199
-
The symmetry of the yeast U6 RNA gene's TATA box and the orientation of the TATA-binding protein in yeast TFIIIB.Genes Dev. 1995 Dec 1;9(23):2974-85. doi: 10.1101/gad.9.23.2974. Genes Dev. 1995. PMID: 7498793
-
Characterization of transcription from TATA-less promoters: identification of a new core promoter element XCPE2 and analysis of factor requirements.PLoS One. 2009;4(4):e5103. doi: 10.1371/journal.pone.0005103. Epub 2009 Apr 1. PLoS One. 2009. PMID: 19337366 Free PMC article.
-
TATA box-mediated in vitro transcription by RNA polymerase III. Evidence for TATA-binding protein in a polymerase III type complex.J Biol Chem. 1993 Jan 15;268(2):1141-50. J Biol Chem. 1993. PMID: 7678250
Cited by
-
The extended AT-hook is a novel RNA binding motif.RNA Biol. 2015;12(8):864-76. doi: 10.1080/15476286.2015.1060394. RNA Biol. 2015. PMID: 26156556 Free PMC article.
-
Conditional knockout mice reveal distinct functions for the global transcriptional coactivators CBP and p300 in T-cell development.Mol Cell Biol. 2006 Feb;26(3):789-809. doi: 10.1128/MCB.26.3.789-809.2006. Mol Cell Biol. 2006. PMID: 16428436 Free PMC article.
-
Surface Glycoproteomic Analysis Reveals That Both Unique and Differential Expression of Surface Glycoproteins Determine the Cell Type.Anal Chem. 2019 May 21;91(10):6934-6942. doi: 10.1021/acs.analchem.9b01447. Epub 2019 May 3. Anal Chem. 2019. PMID: 31025852 Free PMC article.
-
Conserved role of hnRNPL in alternative splicing of epigenetic modifiers enables B cell activation.EMBO Rep. 2024 Jun;25(6):2662-2697. doi: 10.1038/s44319-024-00152-3. Epub 2024 May 14. EMBO Rep. 2024. PMID: 38744970 Free PMC article.
References
-
- Aso, T., H. A. Vasavada, T. Kawaguchi, F. J. Germino, S. Ganguly, S. Kitajima, S. M. Weissman, and Y. Yasukochi. 1992. Characterization of cDNA for the large subunit of the transcription initiation factor TFIIF. Nature 355:461-464. - PubMed
-
- Berger, S. L., and A. R. Kimmel (ed.). 1987. Methods in enzymology: guide to molecular cloning techniques, vol 152. Academic Press, Inc., San Diego, CA. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
