A ferret model of canine distemper virus virulence and immunosuppression
- PMID: 14610181
- PMCID: PMC262577
- DOI: 10.1128/jvi.77.23.12579-12591.2003
A ferret model of canine distemper virus virulence and immunosuppression
Abstract
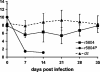
Canine distemper virus (CDV) infects many carnivores, including ferrets and dogs, and is the member of the Morbillivirus genus most easily amenable to experimentation in a homologous small-animal system. To gain insights into the determinants of CDV pathogenesis, we isolated a strain highly virulent for ferrets by repeated passaging in these animals. Sequence comparison of the genome of this strain with that of its highly attenuated precursor revealed 19 mutations distributed almost evenly in the six genes. We then recovered a virus from a cDNA copy of the virulent CDV strain's consensus sequence by using a modified reverse genetics system based on B cells. We infected ferrets with this virus and showed that it fully retained virulence as measured by the timing of rash appearance, disease onset, and death. Body temperature, leukocyte number, lymphocyte proliferation activity, and cell-associated viremia also had similar kinetics. We then addressed the question of the relative importance of the envelope and other viral constituents for virulence. Viruses in which the envelope genes (matrix, fusion, and hemagglutinin) of the virulent strain were combined with the other genes of the attenuated strain caused severe rash and fever even if the disease onset was delayed. Viruses in which the nucleocapsid, polymerase, and phosphoprotein genes (coding also for the V and C proteins) of the virulent strain were combined with the envelope genes of the attenuated strain caused milder signs of disease. Thus, virulence-inducing mutations have accumulated throughout the genome.
Figures

Similar articles
-
Canine Distemper Virus Spread and Transmission to Naive Ferrets: Selective Pressure on Signaling Lymphocyte Activation Molecule-Dependent Entry.J Virol. 2018 Jul 17;92(15):e00669-18. doi: 10.1128/JVI.00669-18. Print 2018 Aug 1. J Virol. 2018. PMID: 29793948 Free PMC article.
-
Rational attenuation of canine distemper virus (CDV) to develop a morbillivirus animal model that mimics measles in humans.J Virol. 2024 Mar 19;98(3):e0185023. doi: 10.1128/jvi.01850-23. Epub 2024 Feb 28. J Virol. 2024. PMID: 38415596 Free PMC article.
-
Persistent and Severe Viral Replication in PBMCs with Moderate Immunosuppression Served an Alternative Novel Pathogenic Mechanism for Canine Morbillivirus.Microbiol Spectr. 2023 Feb 14;11(1):e0406022. doi: 10.1128/spectrum.04060-22. Epub 2022 Dec 19. Microbiol Spectr. 2023. PMID: 36533959 Free PMC article.
-
Morbillivirus Experimental Animal Models: Measles Virus Pathogenesis Insights from Canine Distemper Virus.Viruses. 2016 Oct 11;8(10):274. doi: 10.3390/v8100274. Viruses. 2016. PMID: 27727184 Free PMC article. Review.
-
Ferrets as a model for morbillivirus pathogenesis, complications, and vaccines.Curr Top Microbiol Immunol. 2009;330:73-87. doi: 10.1007/978-3-540-70617-5_4. Curr Top Microbiol Immunol. 2009. PMID: 19203105 Free PMC article. Review.
Cited by
-
Nearby clusters of hemagglutinin residues sustain SLAM-dependent canine distemper virus entry in peripheral blood mononuclear cells.J Virol. 2005 May;79(9):5857-62. doi: 10.1128/JVI.79.9.5857-5862.2005. J Virol. 2005. PMID: 15827201 Free PMC article.
-
Differentiation of CD8+ T cells into effector cells is enhanced by physiological range hyperthermia.J Leukoc Biol. 2011 Nov;90(5):951-62. doi: 10.1189/jlb.0511229. Epub 2011 Aug 26. J Leukoc Biol. 2011. PMID: 21873456 Free PMC article.
-
Canine distemper virus in the Serengeti ecosystem: molecular adaptation to different carnivore species.Mol Ecol. 2017 Apr;26(7):2111-2130. doi: 10.1111/mec.13902. Epub 2016 Dec 7. Mol Ecol. 2017. PMID: 27928865 Free PMC article.
-
Mink SLAM V-Region V74I Substitutions Contribute to the Formation of Syncytia Induced by Canine Distemper Virus.Front Vet Sci. 2021 Jan 21;7:570283. doi: 10.3389/fvets.2020.570283. eCollection 2020. Front Vet Sci. 2021. PMID: 33585591 Free PMC article.
-
Independent structural domains in paramyxovirus polymerase protein.J Biol Chem. 2012 Feb 24;287(9):6878-91. doi: 10.1074/jbc.M111.325258. Epub 2012 Jan 3. J Biol Chem. 2012. PMID: 22215662 Free PMC article.
References
-
- Anonymous. 2002. From the Centers for Disease Control. Measles—United States, 2000. JAMA 287:1105-1106. - PubMed
-
- Aylward, R. B., J. Clements, and J. M. Olive. 1997. The impact of immunization control activities on measles outbreaks in middle and low income countries. Int. J. Epidemiol. 26:662-669. - PubMed
-
- Baranowski, E., C. M. Ruiz-Jarabo, and E. Domingo. 2001. Evolution of cell recognition by viruses. Science 292:1102-1105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

