Expression of Arabidopsis gamma-tubulin in fission yeast reveals conserved and novel functions of gamma-tubulin
- PMID: 14605233
- PMCID: PMC300744
- DOI: 10.1104/pp.103.027367
Expression of Arabidopsis gamma-tubulin in fission yeast reveals conserved and novel functions of gamma-tubulin
Abstract
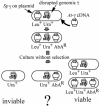
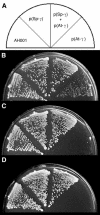
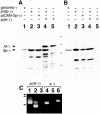
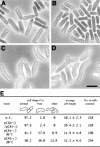
gamma-Tubulin localizes to microtubule-organizing centers in animal and fungal cells where it is important for microtubule nucleation. Plant cells do not have morphologically defined microtubule organizing centers, however, and gamma-tubulin is distributed in small, discrete structures along microtubules. The great difference in distribution has prompted speculation that plant gamma-tubulins function differently from animal and fungal gamma-tubulins. We tested this possibility by expressing Arabidopsis gamma-tubulin in the fission yeast Schizosaccharomyces pombe. At high temperatures, the plant gamma-tubulin was able to bind to microtubule-organizing centers, nucleate microtubule assembly, and support the growth and replication of S. pombe cells lacking endogenous gamma-tubulin. However, the distribution of microtubules was abnormal as was cell morphology, and at low temperatures, cells were arrested in mitosis. These results reveal that Arabidopsis gamma-tubulin can carry out essential functions in S. pombe and is, thus, functionally conserved. The morphological abnormalities reveal that it cannot carry out some nonessential functions, however, and they underscore the importance of gamma-tubulin in morphogenesis of fission yeast cells and in maintaining normal interphase microtubule arrays.
Figures

Similar articles
-
Human gamma-tubulin functions in fission yeast.J Cell Biol. 1994 Sep;126(6):1465-73. doi: 10.1083/jcb.126.6.1465. J Cell Biol. 1994. PMID: 8089179 Free PMC article.
-
Molecular and structural characterization of the spindle pole bodies in the fission yeast Schizosaccharomyces japonicus var japonicus.Yeast. 2002 Nov;19(15):1335-50. doi: 10.1002/yea.921. Yeast. 2002. PMID: 12402243
-
GCP-WD mediates γ-TuRC recruitment and the geometry of microtubule nucleation in interphase arrays of Arabidopsis.Curr Biol. 2014 Nov 3;24(21):2548-55. doi: 10.1016/j.cub.2014.09.013. Epub 2014 Oct 16. Curr Biol. 2014. PMID: 25438942
-
Gamma-tubulins and their functions.Tsitol Genet. 2003 Mar-Apr;37(2):3-10. Tsitol Genet. 2003. PMID: 12774513 Review.
-
Tubulin folding cofactors: half a dozen for a dimer.Curr Biol. 2002 Nov 19;12(22):R767-9. doi: 10.1016/s0960-9822(02)01288-5. Curr Biol. 2002. PMID: 12445400 Review.
Cited by
-
The ring saga: looking back at the discovery of γ-tubulin and γ-tubulin ring complexes.Mol Biol Cell. 2023 Jan 1;34(1):rt1. doi: 10.1091/mbc.E22-07-0290. Mol Biol Cell. 2023. PMID: 36520030 Free PMC article.
-
Gamma-tubulin is essential for acentrosomal microtubule nucleation and coordination of late mitotic events in Arabidopsis.Plant Cell. 2006 May;18(5):1199-212. doi: 10.1105/tpc.105.038364. Epub 2006 Apr 7. Plant Cell. 2006. PMID: 16603653 Free PMC article.
-
Feeding cells induced by phytoparasitic nematodes require γ-tubulin ring complex for microtubule reorganization.PLoS Pathog. 2011 Dec;7(12):e1002343. doi: 10.1371/journal.ppat.1002343. Epub 2011 Dec 1. PLoS Pathog. 2011. PMID: 22144887 Free PMC article.
-
Gamma-tubulin is essential for microtubule organization and development in Arabidopsis.Plant Cell. 2006 Jun;18(6):1412-25. doi: 10.1105/tpc.105.039644. Epub 2006 May 12. Plant Cell. 2006. PMID: 16698945 Free PMC article.
-
Eukaryotic cells and their cell bodies: Cell Theory revised.Ann Bot. 2004 Jul;94(1):9-32. doi: 10.1093/aob/mch109. Epub 2004 May 20. Ann Bot. 2004. PMID: 15155376 Free PMC article. Review.
References
-
- Brinkley BR (1985) Microtubule organizing centers. Annu Rev Cell Biol 1: 145-172 - PubMed
-
- Canaday J, Stoppin-Mellet V, Mutterer J, Lambert AM, Schmit AC (2000) Higher plant cells: γ-tubulin and microtubule nucleation in the absence of centrosomes. Microsci Res Technol 49: 487-495 - PubMed
-
- Ding D-Q, Chikashige Y, Haraguchi T, Hiraoka Y (1998) Oscillatory nuclear movement in fission yeast meiotic prophase is driven by astral microtubules, as revealed by continuous observation of chromosomes and microtubules in living cells. J Cell Sci 111: 701-712 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

