Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors
- PMID: 14605137
- PMCID: PMC262545
- DOI: 10.1128/JCM.41.11.5046-5052.2003
Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors
Abstract
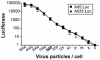
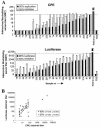
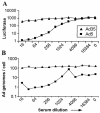
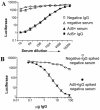
The presence of various levels of anti-adenovirus serotype 5 (Ad5)-neutralizing antibodies in humans is thought to contribute to the inconsistent clinical results obtained so far in diverse gene transfer and vaccination studies and might preclude universal dosing with recombinant Ad5. Prescreening of individuals eligible for Ad5 or alternative serotype treatment and subsequently tailoring the vector dose might aid in ensuring the consistency of clinical parameters. For this purpose, a qualified Ad neutralization assay is required. Here we have tested the different protocols used to date to determine anti-Ad neutralizing activity. Based on simplicity, speed, high throughput, sensitivity, and robustness, we propose a qualified assay in which Ad neutralization is monitored by luciferase reporter gene expression.
Figures

Similar articles
-
Quantitative adenovirus neutralization assays based on the secreted alkaline phosphatase reporter gene: application in epidemiologic studies and in the design of adenovector vaccines.Hum Gene Ther. 2004 Mar;15(3):293-304. doi: 10.1089/104303404322886147. Hum Gene Ther. 2004. PMID: 15018738
-
Circumvention of anti-adenovirus neutralizing immunity by administration of an adenoviral vector of an alternate serotype.Hum Gene Ther. 1997 Jan 1;8(1):99-109. doi: 10.1089/hum.1997.8.1-99. Hum Gene Ther. 1997. PMID: 8989999
-
Induction of protective immunity to anthrax lethal toxin with a nonhuman primate adenovirus-based vaccine in the presence of preexisting anti-human adenovirus immunity.Infect Immun. 2005 Oct;73(10):6885-91. doi: 10.1128/IAI.73.10.6885-6891.2005. Infect Immun. 2005. PMID: 16177368 Free PMC article.
-
Adenovirus vectors composed of subgroup B adenoviruses.Curr Gene Ther. 2007 Aug;7(4):229-38. doi: 10.2174/156652307781369137. Curr Gene Ther. 2007. PMID: 17969556 Review.
-
[Development of a replication-incompetent adenovirus vector derived from subgroup B adenovirus serotype 35].Yakugaku Zasshi. 2008 Dec;128(12):1751-61. doi: 10.1248/yakushi.128.1751. Yakugaku Zasshi. 2008. PMID: 19043294 Review. Japanese.
Cited by
-
Optimization of Spray Drying Conditions for Yield, Particle Size and Biological Activity of Thermally Stable Viral Vectors.Pharm Res. 2016 Nov;33(11):2763-76. doi: 10.1007/s11095-016-2003-4. Epub 2016 Jul 22. Pharm Res. 2016. PMID: 27450412
-
Disrupted adenovirus-based vaccines against small addictive molecules circumvent anti-adenovirus immunity.Hum Gene Ther. 2013 Jan;24(1):58-66. doi: 10.1089/hum.2012.163. Epub 2012 Dec 21. Hum Gene Ther. 2013. PMID: 23140508 Free PMC article.
-
Human adenovirus 5-vectored Plasmodium falciparum NMRC-M3V-Ad-PfCA vaccine encoding CSP and AMA1 is safe, well-tolerated and immunogenic but does not protect against controlled human malaria infection.Hum Vaccin Immunother. 2013 Oct;9(10):2165-77. doi: 10.4161/hv.24941. Epub 2013 Jun 4. Hum Vaccin Immunother. 2013. PMID: 23899517 Free PMC article. Clinical Trial.
-
A novel high-throughput vaccinia virus neutralization assay and preexisting immunity in populations from different geographic regions in China.PLoS One. 2012;7(3):e33392. doi: 10.1371/journal.pone.0033392. Epub 2012 Mar 16. PLoS One. 2012. PMID: 22438922 Free PMC article.
-
Immunogenicity of heterologous recombinant adenovirus prime-boost vaccine regimens is enhanced by circumventing vector cross-reactivity.J Virol. 2006 Dec;80(24):12009-16. doi: 10.1128/JVI.01749-06. Epub 2006 Oct 11. J Virol. 2006. PMID: 17035318 Free PMC article.
References
-
- Bruna-Romero, O., G. Gonzalez-Aseguinolaza, J. C. Hafalla, M. Tsuji, and R. S. Nussenzweig. 2001. Complete, long-lasting protection against malaria of mice primed and boosted with two distinct viral vectors expressing the same plasmodial antigen. Proc. Natl. Acad. Sci. USA 98:11491-11496. - PMC - PubMed
-
- Chen, Y., D. C. Yu, D. Charlton, and D. R. Henderson. 2000. Pre-existent adenovirus antibody inhibits systemic toxicity and antitumor activity of CN706 in the nude mouse LNCaP xenograft model: implications and proposals for human therapy. Hum. Gene Ther. 11:1553-1567. - PubMed
-
- Fallaux, F. J., A. Bout, I. van der Velde, D. J. van den Wollenberg, K. M. Hehir, J. Keegan, C. Auger, S. J. Cramer, H. van Ormondt, A. J. van der Eb, D. Valerio, and R. C. Hoeben. 1998. New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenoviruses. Hum. Gene Ther. 9:1909-1917. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

