The Rab8 GTPase selectively regulates AP-1B-dependent basolateral transport in polarized Madin-Darby canine kidney cells
- PMID: 14581456
- PMCID: PMC2173525
- DOI: 10.1083/jcb.200307046
The Rab8 GTPase selectively regulates AP-1B-dependent basolateral transport in polarized Madin-Darby canine kidney cells
Abstract
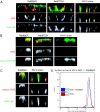
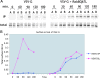
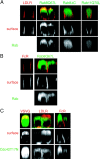
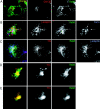
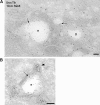
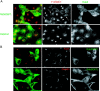
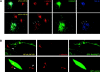
The AP-1B clathrin adaptor complex plays a key role in the recognition and intracellular transport of many membrane proteins destined for the basolateral surface of epithelial cells. However, little is known about other components that act in conjunction with AP-1B. We found that the Rab8 GTPase is one such component. Expression of a constitutively activated GTP hydrolysis mutant selectively inhibited basolateral (but not apical) transport of newly synthesized membrane proteins. Moreover, the effects were limited to AP-1B-dependent basolateral cargo; basolateral transport of proteins containing dileucine targeting motifs that do not interact with AP-1B were targeted normally despite overexpression of mutant Rab8. Similar results were obtained for a dominant-negative allele of the Rho GTPase Cdc42, previously implicated in basolateral transport but now shown to be selective for the AP-1B pathway. Rab8-GFP was localized to membranes in the TGN-recycling endosome, together with AP-1B complexes and the closely related but ubiquitously expressed AP-1A complex. However, expression of active Rab8 caused a selective dissociation of AP-1B complexes, reflecting the specificity of Rab8 for AP-1B-dependent transport.
Figures
Similar articles
-
The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains.J Cell Biol. 2003 Oct 27;163(2):351-62. doi: 10.1083/jcb.200309020. J Cell Biol. 2003. PMID: 14581457 Free PMC article.
-
Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells.J Cell Biol. 2001 Feb 5;152(3):595-606. doi: 10.1083/jcb.152.3.595. J Cell Biol. 2001. PMID: 11157985 Free PMC article.
-
Rab10 is involved in basolateral transport in polarized Madin-Darby canine kidney cells.Traffic. 2007 Jan;8(1):47-60. doi: 10.1111/j.1600-0854.2006.00506.x. Epub 2006 Nov 21. Traffic. 2007. PMID: 17132146
-
The building blocks for basolateral vesicles in polarized epithelial cells.Trends Cell Biol. 2005 Apr;15(4):222-8. doi: 10.1016/j.tcb.2005.02.006. Trends Cell Biol. 2005. PMID: 15817379 Review.
-
Role of the epithelial cell-specific clathrin adaptor complex AP-1B in cell polarity.Cell Logist. 2015 Jul 30;5(2):e1074331. doi: 10.1080/21592799.2015.1074331. eCollection 2015 Apr-Jun. Cell Logist. 2015. PMID: 27057418 Free PMC article. Review.
Cited by
-
Ehbp1 orchestrates orderly sorting of Wnt/Wingless to the basolateral and apical cell membranes.EMBO Rep. 2024 Oct 14. doi: 10.1038/s44319-024-00289-1. Online ahead of print. EMBO Rep. 2024. PMID: 39402333
-
Viral and cellular determinants of polarized trafficking of viral envelope proteins from insect-specific and insect-vectored viruses in insect midgut and salivary gland cells.J Virol. 2024 Sep 17;98(9):e0054024. doi: 10.1128/jvi.00540-24. Epub 2024 Aug 20. J Virol. 2024. PMID: 39162433
-
Receptor-mediated internalization promotes increased endosome size and number in a RAB4- and RAB5-dependent manner.Eur J Cell Biol. 2023 Sep;102(3):151339. doi: 10.1016/j.ejcb.2023.151339. Epub 2023 Jul 6. Eur J Cell Biol. 2023. PMID: 37423034 Free PMC article.
-
AP-1 Recruits SMAP-1/SMAPs to the trans-Golgi Network to Promote Sorting in Polarized Epithelia.Front Cell Dev Biol. 2021 Nov 25;9:774401. doi: 10.3389/fcell.2021.774401. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34901019 Free PMC article.
-
A Brucella effector modulates the Arf6-Rab8a GTPase cascade to promote intravacuolar replication.EMBO J. 2021 Oct 1;40(19):e107664. doi: 10.15252/embj.2021107664. Epub 2021 Aug 23. EMBO J. 2021. PMID: 34423453 Free PMC article.
References
-
- Carroll, K.S., J. Hanna, I. Simon, J. Krise, P. Barbero, and S.R. Pfeffer. 2001. Role of Rab9 GTPase in facilitating receptor recruitment by TIP47. Science. 292:1373–1376. - PubMed
-
- Cohen, D., A. Musch, and E. Rodriguez-Boulan. 2001. Selective control of basolateral membrane protein polarity by cdc42. Traffic. 2:556–564. - PubMed
-
- Drubin, D.G., and W.J. Nelson. 1996. Origins of cell polarity. Cell. 84:335–344. - PubMed
-
- Erickson, J.W., C. Zhang, R.A. Kahn, T. Evans, and R.A. Cerione. 1996. Mammalian Cdc42 is a brefeldin A-sensitive component of the Golgi apparatus. J. Biol. Chem. 271:26850–26854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

