Myosin-Va binds to and mechanochemically couples microtubules to actin filaments
- PMID: 14565972
- PMCID: PMC307536
- DOI: 10.1091/mbc.e03-07-0504
Myosin-Va binds to and mechanochemically couples microtubules to actin filaments
Abstract
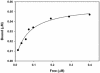
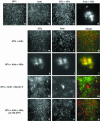
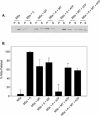
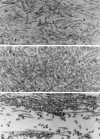
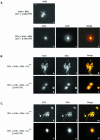
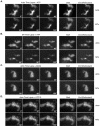
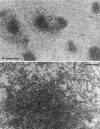
Myosin-Va was identified as a microtubule binding protein by cosedimentation analysis in the presence of microtubules. Native myosin-Va purified from chick brain, as well as the expressed globular tail domain of this myosin, but not head domain bound to microtubule-associated protein-free microtubules. Binding of myosin-Va to microtubules was saturable and of moderately high affinity (approximately 1:24 Myosin-Va:tubulin; Kd = 70 nM). Myosin-Va may bind to microtubules via its tail domain because microtubule-bound myosin-Va retained the ability to bind actin filaments resulting in the formation of cross-linked gels of microtubules and actin, as assessed by fluorescence and electron microscopy. In low Ca2+, ATP addition induced dissolution of these gels, but not release of myosin-Va from MTs. However, in 10 microM Ca2+, ATP addition resulted in the contraction of the gels into aster-like arrays. These results demonstrate that myosin-Va is a microtubule binding protein that cross-links and mechanochemically couples microtubules to actin filaments.
Figures
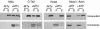

Similar articles
-
Myosin Va bound to phagosomes binds to F-actin and delays microtubule-dependent motility.Mol Biol Cell. 2001 Sep;12(9):2742-55. doi: 10.1091/mbc.12.9.2742. Mol Biol Cell. 2001. PMID: 11553713 Free PMC article.
-
High affinity binding of brain myosin-Va to F-actin induced by calcium in the presence of ATP.J Biol Chem. 2001 Oct 26;276(43):39812-8. doi: 10.1074/jbc.M102583200. Epub 2001 Aug 21. J Biol Chem. 2001. PMID: 11517216
-
The tail domain of myosin Va modulates actin binding to one head.J Biol Chem. 2006 Oct 20;281(42):31326-36. doi: 10.1074/jbc.M603898200. Epub 2006 Aug 18. J Biol Chem. 2006. PMID: 16921171
-
Vesicle transport: the role of actin filaments and myosin motors.Microsc Res Tech. 1999 Oct 15;47(2):93-106. doi: 10.1002/(SICI)1097-0029(19991015)47:2<93::AID-JEMT2>3.0.CO;2-P. Microsc Res Tech. 1999. PMID: 10523788 Review.
-
Myosin-V motility: these levers were made for walking.Trends Cell Biol. 2003 Sep;13(9):447-51. doi: 10.1016/s0962-8924(03)00172-7. Trends Cell Biol. 2003. PMID: 12946621 Review.
Cited by
-
A CRM1-mediated nuclear export signal is essential for cytoplasmic localization of neurogenin 3 in neurons.PLoS One. 2013;8(1):e55237. doi: 10.1371/journal.pone.0055237. Epub 2013 Jan 30. PLoS One. 2013. PMID: 23383123 Free PMC article.
-
Slip sliding away with myosin V.Proc Natl Acad Sci U S A. 2007 Mar 27;104(13):5255-6. doi: 10.1073/pnas.0701071104. Epub 2007 Mar 20. Proc Natl Acad Sci U S A. 2007. PMID: 17374717 Free PMC article. No abstract available.
-
Myosin V and Kinesin act as tethers to enhance each others' processivity.Proc Natl Acad Sci U S A. 2008 Mar 25;105(12):4691-6. doi: 10.1073/pnas.0711531105. Epub 2008 Mar 17. Proc Natl Acad Sci U S A. 2008. PMID: 18347333 Free PMC article.
-
Cell non-autonomy amplifies disruption of neurulation by mosaic Vangl2 deletion in mice.Nat Commun. 2021 Feb 19;12(1):1159. doi: 10.1038/s41467-021-21372-4. Nat Commun. 2021. PMID: 33608529 Free PMC article.
-
Defects in Axonal Transport in Inherited Neuropathies.J Neuromuscul Dis. 2019;6(4):401-419. doi: 10.3233/JND-190427. J Neuromuscul Dis. 2019. PMID: 31561383 Free PMC article. Review.
References
-
- Burns, R.G., and Islam, K. (1984). Stoichiometry of microtubule-associated protein (MAP2):tubulin and the localisation of the phosphorylation and cysteine residues along the MAP2 primary sequence. Eur. J. Biochem. 141, 599–608. - PubMed
-
- Cheney, R.E. (1998). Purification and assay of myosin V. Methods Enzymol. 298, 3–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

