Molecular mechanism for a role of SHP2 in epidermal growth factor receptor signaling
- PMID: 14560030
- PMCID: PMC207628
- DOI: 10.1128/MCB.23.21.7875-7886.2003
Molecular mechanism for a role of SHP2 in epidermal growth factor receptor signaling
Abstract
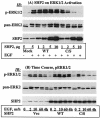
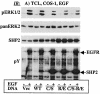
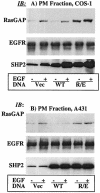
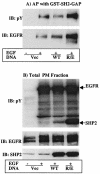
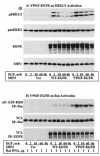
The Src homology 2-containing phosphotyrosine phosphatase (SHP2) is primarily a positive effector of receptor tyrosine kinase signaling. However, the molecular mechanism by which SHP2 effects its biological function is unknown. In this report, we provide evidence that defines the molecular mechanism and site of action of SHP2 in the epidermal growth factor-induced mitogenic pathway. We demonstrate that SHP2 acts upstream of Ras and functions by increasing the half-life of activated Ras (GTP-Ras) in the cell by interfering with the process of Ras inactivation catalyzed by Ras GTPase-activating protein (RasGAP). It does so by inhibition of tyrosine phosphorylation-dependent translocation of RasGAP to the plasma membrane, to its substrate (GTP-Ras) microdomain. Inhibition is achieved through the dephosphorylation of RasGAP binding sites at the level of the plasma membrane. We have identified Tyr992 of the epidermal growth factor receptor (EGFR) to be one such site, since its mutation to Phe renders the EGFR refractory to the effect of dominant-negative SHP2. To our knowledge, this is the first report to outline the site and molecular mechanism of action of SHP2 in EGFR signaling, which may also serve as a model to describe its role in other receptor tyrosine kinase signaling pathways.
Figures


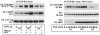



Similar articles
-
A novel role for Gab1 and SHP2 in epidermal growth factor-induced Ras activation.J Biol Chem. 2005 Feb 18;280(7):5350-60. doi: 10.1074/jbc.M410012200. Epub 2004 Dec 1. J Biol Chem. 2005. PMID: 15574420
-
A specific amino acid context in EGFR and HER2 phosphorylation sites enables selective binding to the active site of Src homology phosphatase 2 (SHP2).J Biol Chem. 2020 Mar 13;295(11):3563-3575. doi: 10.1074/jbc.RA119.011422. Epub 2020 Feb 4. J Biol Chem. 2020. PMID: 32024694 Free PMC article.
-
A critical role for phosphoinositide 3-kinase upstream of Gab1 and SHP2 in the activation of ras and mitogen-activated protein kinases by epidermal growth factor.J Biol Chem. 2001 Mar 23;276(12):8856-64. doi: 10.1074/jbc.M006966200. Epub 2000 Dec 27. J Biol Chem. 2001. PMID: 11134009
-
Shp2 regulates SRC family kinase activity and Ras/Erk activation by controlling Csk recruitment.Mol Cell. 2004 Feb 13;13(3):341-55. doi: 10.1016/s1097-2765(04)00050-4. Mol Cell. 2004. PMID: 14967142
-
The molecular functions of Shp2 in the Ras/Mitogen-activated protein kinase (ERK1/2) pathway.Cell Signal. 2008 Mar;20(3):453-9. doi: 10.1016/j.cellsig.2007.10.002. Epub 2007 Oct 11. Cell Signal. 2008. PMID: 17993263 Review.
Cited by
-
Redox regulation of epidermal growth factor receptor signaling through cysteine oxidation.Biochemistry. 2012 Dec 18;51(50):9954-65. doi: 10.1021/bi301441e. Epub 2012 Dec 5. Biochemistry. 2012. PMID: 23186290 Free PMC article. Review.
-
Allosteric SHP2 Inhibitor, IACS-13909, Overcomes EGFR-Dependent and EGFR-Independent Resistance Mechanisms toward Osimertinib.Cancer Res. 2020 Nov 1;80(21):4840-4853. doi: 10.1158/0008-5472.CAN-20-1634. Epub 2020 Sep 14. Cancer Res. 2020. PMID: 32928921 Free PMC article.
-
S-nitrosylated SHP-2 contributes to NMDA receptor-mediated excitotoxicity in acute ischemic stroke.Proc Natl Acad Sci U S A. 2013 Feb 19;110(8):3137-42. doi: 10.1073/pnas.1215501110. Epub 2013 Feb 4. Proc Natl Acad Sci U S A. 2013. PMID: 23382182 Free PMC article.
-
Src homology 2-containing phosphotyrosine phosphatase regulates endothelin-1-induced epidermal growth factor receptor transactivation in rat renal tubular cell NRK-52E.Pflugers Arch. 2006 Apr;452(1):16-24. doi: 10.1007/s00424-005-0006-9. Epub 2005 Oct 28. Pflugers Arch. 2006. PMID: 16261333
-
Modulation of the tumor suppressor protein alpha-catenin by ischemic microenvironment.Am J Pathol. 2009 Oct;175(4):1662-74. doi: 10.2353/ajpath.2009.090007. Epub 2009 Sep 10. Am J Pathol. 2009. PMID: 19745064 Free PMC article.
References
-
- Agazie, Y. M., and M. J. Hayman. Oncogene, in press. - PubMed
-
- Agazie, Y. M., and M. J. Hayman. 2003. Development of an efficient “substrate-trapping” mutant of Src homology phosphotyrosine phosphatase 2 and identification of the epidermal growth factor receptor, Gab1, and three other proteins as target substrates. J. Biol. Chem. 278:13952-13958. - PubMed
-
- Bardelli, A., P. Longati, D. Gramaglia, M. C. Stella, and P. M. Comoglio. 1997. Gab1 coupling to the HGF/Met receptor multifunctional docking site requires binding of Grb2 and correlates with the transforming potential. Oncogene 15:3103-3111. - PubMed
-
- Carlberg, K., and L. R. Rohrschneider. 1997. Characterization of a novel tyrosine phosphorylated 100-kDa protein that binds to SHP-2 and phosphatidylinositol 3′-kinase in myeloid cells. J. Biol. Chem. 272:15943-15950. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
