Polyclonal immunoglobulin G from patients neutralizes human immunodeficiency virus type 1 primary isolates by binding free virions, but without interfering with an initial CD4-independent attachment of the virus to primary blood mononuclear cells
- PMID: 14557624
- PMCID: PMC229376
- DOI: 10.1128/jvi.77.21.11385-11397.2003
Polyclonal immunoglobulin G from patients neutralizes human immunodeficiency virus type 1 primary isolates by binding free virions, but without interfering with an initial CD4-independent attachment of the virus to primary blood mononuclear cells
Abstract
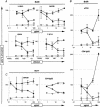
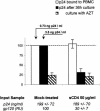
We investigated the relationship between human immunodeficiency virus type 1 (HIV-1) primary isolate (PI) antibody-mediated neutralization and attachment to primary blood mononuclear cells (PBMC). Incubation of PIs with immunoglobulin G (IgG) purified from infected patients did not inhibit attachment of the viruses with PBMC, but partial to complete neutralization was achieved. Neutralization of PIs already fixed on the cells was achieved by some IgG samples only and was of limited intensity compared to the former neutralization protocol. On the contrary, the binding of IgG to free virions was shown to be sufficient to reach potent neutralization, as the infectivity of IgG-PI complexes purified from the bulk of antibodies before addition to PBMC was strongly diminished compared to mock-treated controls. Monoclonal antibodies to the CDR2 domain of CD4 completely inhibited the infection of PBMC without interfering with the attachment of PIs to the cells, suggesting that, under these experimental conditions, the initial attachment of viruses to PBMC involves alternative cellular receptors. This initial interaction may also involve other components of the viral envelope than gp120, as partial depletion of the surface glycoproteins of primary viral particles that resulted in an almost complete loss of infectivity did not impair attachment to PBMC. A limited inhibition of attachment was observed when interfering with putative interactions with cellular heparan sulfate, whereas no effect was observed for cellular CD147 or nucleolin or for virion-incorporated cyclophilin A. Altogether, our results favor a mechanism of neutralization of HIV-1 PIs by polyclonal IgG where antibodies predominantly bind free virions and neutralize without interfering with the attachment to PBMC, which, in this model, is mainly CD4 independent.
Figures
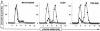

Similar articles
-
Recombinant human monoclonal antibody IgG1b12 neutralizes diverse human immunodeficiency virus type 1 primary isolates.AIDS Res Hum Retroviruses. 1997 May 1;13(7):575-82. doi: 10.1089/aid.1997.13.575. AIDS Res Hum Retroviruses. 1997. PMID: 9135875
-
Binding of antibodies to virion-associated gp120 molecules of primary-like human immunodeficiency virus type 1 (HIV-1) isolates: effect on HIV-1 infection of macrophages and peripheral blood mononuclear cells.Virology. 1997 Mar 17;229(2):360-9. doi: 10.1006/viro.1997.8443. Virology. 1997. PMID: 9126249
-
Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates.J Virol. 1992 Jan;66(1):235-43. doi: 10.1128/JVI.66.1.235-243.1992. J Virol. 1992. PMID: 1727487 Free PMC article.
-
HIV-1 attachment: another look.Trends Microbiol. 1999 Apr;7(4):144-9. doi: 10.1016/s0966-842x(99)01474-2. Trends Microbiol. 1999. PMID: 10217828 Review.
-
Occupancy and mechanism in antibody-mediated neutralization of animal viruses.J Gen Virol. 2002 Sep;83(Pt 9):2091-2108. doi: 10.1099/0022-1317-83-9-2091. J Gen Virol. 2002. PMID: 12185262 Review.
Cited by
-
Efficient inhibition of HIV-1 replication in human immature monocyte-derived dendritic cells by purified anti-HIV-1 IgG without induction of maturation.Blood. 2006 Jun 1;107(11):4466-74. doi: 10.1182/blood-2005-08-3490. Epub 2006 Feb 9. Blood. 2006. PMID: 16469871 Free PMC article.
-
Time frames for neutralization during the human immunodeficiency virus type 1 entry phase, as monitored in synchronously infected cell cultures.J Virol. 2007 Apr;81(7):3525-34. doi: 10.1128/JVI.02293-06. Epub 2007 Jan 24. J Virol. 2007. PMID: 17251303 Free PMC article.
-
Inhibition of HIV Env binding to cellular receptors by monoclonal antibody 2G12 as probed by Fc-tagged gp120.Retrovirology. 2006 Jul 3;3:39. doi: 10.1186/1742-4690-3-39. Retrovirology. 2006. PMID: 16817962 Free PMC article.
-
Stimulation of HIV-1 replication in immature dendritic cells in contact with primary CD4 T or B lymphocytes.J Virol. 2010 May;84(9):4172-82. doi: 10.1128/JVI.01567-09. Epub 2010 Feb 10. J Virol. 2010. PMID: 20147388 Free PMC article.
-
Interactions between natural killer cells and antibody Fc result in enhanced antibody neutralization of human immunodeficiency virus type 1.J Virol. 2005 Feb;79(4):2042-9. doi: 10.1128/JVI.79.4.2042-2049.2005. J Virol. 2005. PMID: 15681406 Free PMC article.
References
-
- Allaway, G. P., K. L. Davis-Bruno, G. A. Beaudry, E. B. Garcia, E. L. Wong, A. M. Ryder, K. W. Hasel, M. C. Gauduin, R. A. Koup, J. S. McDougal, et al. 1995. Expression and characterization of CD4-IgG2, a novel heterotetramer that neutralizes primary HIV type 1 isolates. AIDS Res. Hum. Retrovir. 11:533-539. - PubMed
-
- Beirnaert, E., S. De Zutter, W. Janssens, and G. G. van der Groen. 2001. Potent broad cross-neutralizing sera inhibit attachment of primary HIV-1 isolates (groups M and O) to peripheral blood mononuclear cells. Virology 281:305-314. - PubMed
-
- Binley, J. M., A. Trkola, T. Ketas, D. Schiller, B. Clas, S. Little, D. Richman, A. Hurley, M. Markowitz, and J. P. Moore. 2000. The effect of highly active antiretroviral therapy on binding and neutralizing antibody responses to human immunodeficiency virus type 1 infection. J. Infect. Dis. 182:945-949. - PubMed
-
- Blanco, J., J. Barretina, A. Gutierrez, M. Armand-Ugon, C. Cabrera, B. Clotet, and J. A. Este. 2002. Preferential attachment of HIV particles to activated and CD45RO+CD4+ T cells. AIDS Res. Hum. Retrovir. 18:27-38. - PubMed
-
- Bounou, S., N. Dumais, and M. J. Tremblay. 2001. Attachment of human immunodeficiency virus-1 (HIV-1) particles bearing host-encoded B7-2 proteins leads to nuclear factor-kappa B- and nuclear factor of activated T cells-dependent activation of HIV-1 long terminal repeat transcription. J. Biol. Chem. 276:6359-6369. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

