Inhibition of translation and induction of apoptosis by Bunyaviral nonstructural proteins bearing sequence similarity to reaper
- PMID: 14517326
- PMCID: PMC207008
- DOI: 10.1091/mbc.e03-03-0139
Inhibition of translation and induction of apoptosis by Bunyaviral nonstructural proteins bearing sequence similarity to reaper
Abstract
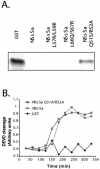
Members of the California serogroup of bunyaviruses (family Bunyaviridae) are the leading cause of pediatric viral encephalitis in North America. Significant cell death is observed as part of the infection pathology. We now report that a Bunyaviral nonstructural protein termed NSs shows sequence similarity to Reaper, a proapoptotic protein from Drosophila. Although NSs proteins lack the Reaper N-terminal motif critical for IAP inhibition, they do retain other functions of Reaper that map to conserved C-terminal regions. Like Reaper, NSs proteins induce mitochondrial cytochrome c release and caspase activation in cell-free extracts and promote neuronal apoptosis and mortality in a mouse model. Independent of caspase activation, Bunyavirus NSs proteins also share with Reaper the ability to directly inhibit cellular protein translation. We have found that the shared capacity to inhibit translation and induce apoptosis resides in common sequence motifs present in both Reaper and NSs proteins. Data presented here suggest that NSs induce apoptosis through a mechanism similar to that used by Reaper, as both proteins bind to an apoptotic regulator called Scythe and can relieve Scythe inhibition of Hsp70. Thus, bunyavirus NSs proteins have multiple Reaper-like functions that likely contribute to viral pathogenesis by promoting cell death and/or inhibiting cellular translation.
Figures




Similar articles
-
Scythe: a novel reaper-binding apoptotic regulator.EMBO J. 1998 Nov 2;17(21):6135-43. doi: 10.1093/emboj/17.21.6135. EMBO J. 1998. PMID: 9799223 Free PMC article.
-
Reaper-induced dissociation of a Scythe-sequestered cytochrome c-releasing activity.EMBO J. 1999 Oct 15;18(20):5486-93. doi: 10.1093/emboj/18.20.5486. EMBO J. 1999. PMID: 10523293 Free PMC article.
-
Reversible inhibition of Hsp70 chaperone function by Scythe and Reaper.EMBO J. 2001 Mar 1;20(5):1033-41. doi: 10.1093/emboj/20.5.1033. EMBO J. 2001. PMID: 11230127 Free PMC article.
-
Viral modulators of cell death provide new links to old pathways.Curr Opin Cell Biol. 2003 Dec;15(6):700-5. doi: 10.1016/j.ceb.2003.10.007. Curr Opin Cell Biol. 2003. PMID: 14644194 Review.
-
Apoptosis in Drosophila: which role for mitochondria?Apoptosis. 2016 Mar;21(3):239-51. doi: 10.1007/s10495-015-1209-y. Apoptosis. 2016. PMID: 26679112 Review.
Cited by
-
NSm protein of Rift Valley fever virus suppresses virus-induced apoptosis.J Virol. 2007 Dec;81(24):13335-45. doi: 10.1128/JVI.01238-07. Epub 2007 Oct 3. J Virol. 2007. PMID: 17913816 Free PMC article.
-
Bag6/Bat3/Scythe: a novel chaperone activity with diverse regulatory functions in protein biogenesis and degradation.Bioessays. 2013 Apr;35(4):377-85. doi: 10.1002/bies.201200159. Epub 2013 Feb 18. Bioessays. 2013. PMID: 23417671 Free PMC article. Review.
-
A Look into Bunyavirales Genomes: Functions of Non-Structural (NS) Proteins.Viruses. 2021 Feb 18;13(2):314. doi: 10.3390/v13020314. Viruses. 2021. PMID: 33670641 Free PMC article. Review.
-
Crimean-Congo hemorrhagic fever virus-infected hepatocytes induce ER-stress and apoptosis crosstalk.PLoS One. 2012;7(1):e29712. doi: 10.1371/journal.pone.0029712. Epub 2012 Jan 6. PLoS One. 2012. PMID: 22238639 Free PMC article.
-
The Andes hantavirus NSs protein is expressed from the viral small mRNA by a leaky scanning mechanism.J Virol. 2012 Feb;86(4):2176-87. doi: 10.1128/JVI.06223-11. Epub 2011 Dec 7. J Virol. 2012. PMID: 22156529 Free PMC article.
References
-
- Anderson, J.R. (2001). The mechanisms of direct, virus-induced destruction of neurons. Curr. Top. Microbiol. Immunol. 253, 15–33. - PubMed
-
- Baldi, P., Brunak, S., Fransconi, P., Pollastri, G., and Soda, G. (1999). Exploiting the past and the future in protein secondary structure prediction. Bioinformatics 15, 937–946. - PubMed
-
- Bangs, P., and White, K. (2000). Regulation and execution of apoptosis during Drosophila development. Dev. Dyn. 218, 68–79. - PubMed
-
- Benedict, C.A., Norris, P.S., and Ware, C.F. (2002). To kill or be killed: viral evasion of apoptosis. Nat. Immunol. 3, 1013–1018. - PubMed
-
- Bergmann, A., Agapite, J., and Steller, H. (1998). Mechanisms and control of programmed cell death in invertebrates. Oncogene 17, 3215–3223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

