Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival
- PMID: 14517300
- PMCID: PMC230333
- DOI: 10.1128/MCB.23.20.7315-7328.2003
Akt-directed glucose metabolism can prevent Bax conformation change and promote growth factor-independent survival
Abstract
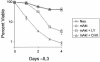
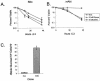
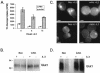
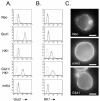
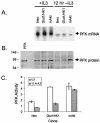
The serine/threonine kinase Akt is a component of many receptor signal transduction pathways and can prevent cell death following growth factor withdrawal. Here, we show that Akt inhibition of cell death is not dependent on new protein translation. Instead, Akt inhibition of cell death requires glucose hydrolysis through glycolysis. Akt was found to regulate multiple steps in glycolysis via posttranscriptional mechanisms that included localization of the glucose transporter, Glut1, to the cell surface and maintenance of hexokinase function in the absence of extrinsic factors. To test the role of glucose uptake and phosphorylation in growth factor-independent survival, cells were transfected with Glut1 and hexokinase 1 (Glut1/HK1) cells. Glut1/HK1 cells accumulated Glut1 on the cell surface and had high glucose uptake capacity similar to that of cells with constitutively active Akt (mAkt). Unlike mAkt-expressing cells, however, they did not consume more glucose, did not maintain prolonged phosphofructokinase-1 protein levels and activity, and did not maintain pentose phosphate shuttle activity in the absence of growth factor. Nevertheless, expression of Glut1 and HK1 promoted increased cytosolic NADH and NADPH levels relative to those of the control cells upon growth factor withdrawal, prevented activation of Bax, and promoted growth factor-independent survival. These data indicate that Bax conformation is sensitive to glucose metabolism and that maintaining glucose uptake and phosphorylation can promote cell survival in the absence of growth factor. Furthermore, Akt required glucose and the ability to perform glycolysis to prevent Bax activation. The prevention of Bax activation by posttranscriptional regulation of glucose metabolism may, therefore, be a required aspect of the ability of Akt to maintain long-term cell survival in the absence of growth factors.
Figures
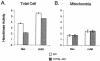



Similar articles
-
Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking.Mol Biol Cell. 2007 Apr;18(4):1437-46. doi: 10.1091/mbc.e06-07-0593. Epub 2007 Feb 14. Mol Biol Cell. 2007. PMID: 17301289 Free PMC article.
-
Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake.Mol Biol Cell. 2002 Jul;13(7):2276-88. doi: 10.1091/mbc.01-12-0584. Mol Biol Cell. 2002. PMID: 12134068 Free PMC article.
-
Interleukin-3-mediated cell survival signals include phosphatidylinositol 3-kinase-dependent translocation of the glucose transporter GLUT1 to the cell surface.J Biol Chem. 2003 Oct 10;278(41):39337-48. doi: 10.1074/jbc.M305689200. Epub 2003 Jul 16. J Biol Chem. 2003. PMID: 12869574
-
A role for the serine/threonine kinase, Akt, in insulin-stimulated glucose uptake.Biochem Soc Trans. 1997 Aug;25(3):981-8. doi: 10.1042/bst0250981. Biochem Soc Trans. 1997. PMID: 9388586 Review. No abstract available.
-
Metabolic phenotype of bladder cancer.Cancer Treat Rev. 2016 Apr;45:46-57. doi: 10.1016/j.ctrv.2016.03.005. Epub 2016 Mar 8. Cancer Treat Rev. 2016. PMID: 26975021 Review.
Cited by
-
Tumor mechanics and metabolic dysfunction.Free Radic Biol Med. 2015 Feb;79:269-80. doi: 10.1016/j.freeradbiomed.2014.11.020. Epub 2014 Dec 19. Free Radic Biol Med. 2015. PMID: 25532934 Free PMC article. Review.
-
Matrix metalloproteinase 11 protects from diabesity and promotes metabolic switch.Sci Rep. 2016 Apr 29;6:25140. doi: 10.1038/srep25140. Sci Rep. 2016. PMID: 27126782 Free PMC article.
-
Mitochondrial hexokinase II (HKII) and phosphoprotein enriched in astrocytes (PEA15) form a molecular switch governing cellular fate depending on the metabolic state.Proc Natl Acad Sci U S A. 2012 Jan 31;109(5):1518-23. doi: 10.1073/pnas.1108225109. Epub 2012 Jan 10. Proc Natl Acad Sci U S A. 2012. PMID: 22233811 Free PMC article.
-
Hypoxia and classical activation limits Mycobacterium tuberculosis survival by Akt-dependent glycolytic shift in macrophages.Cell Death Discov. 2016 May 30;2:16022. doi: 10.1038/cddiscovery.2016.22. eCollection 2016. Cell Death Discov. 2016. PMID: 27551515 Free PMC article.
-
AKT1 phosphorylation of cytoplasmic ME2 induces a metabolic switch to glycolysis for tumorigenesis.Nat Commun. 2024 Jan 23;15(1):686. doi: 10.1038/s41467-024-44772-8. Nat Commun. 2024. PMID: 38263319 Free PMC article.
References
-
- Alessi, D. R., S. R. James, C. P. Downes, A. B. Holmes, P. R. Gaffney, C. B. Reese, and P. Cohen. 1997. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 7:261-269. - PubMed
-
- Atasoy, U., J. Watson, D. Patel, and J. D. Keene. 1998. ELAV protein HuA (HuR) can redistribute between nucleus and cytoplasm and is upregulated during serum stimulation and T cell activation. J. Cell Sci. 111:3145-3156. - PubMed
-
- Baldwin, S. A., L. F. Barros, and M. Griffiths. 1995. Trafficking of glucose transporters—signals and mechanisms. Biosci. Rep. 15:419-426. - PubMed
-
- Barthel, A., S. T. Okino, J. Liao, K. Nakatani, J. Li, J. P. J. Whitlock, and R. A. Roth. 1999. Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1. J. Biol. Chem. 274:20281-20286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
