Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones
- PMID: 14500767
- PMCID: PMC2343578
- DOI: 10.1113/jphysiol.2003.052639
Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones
Abstract
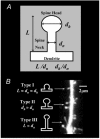
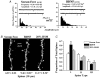
The refinement and plasticity of neuronal connections require synaptic activity and neurotrophin signalling; their specific contributions and interplay are, however, poorly understood. We show here that brain-derived neurotrophic factor (BDNF) increased spine density in apical dendrites of CA1 pyramidal neurones in organotypic slice cultures prepared from postnatal rat hippocampal slices. This effect was observed also in the absence of action potentials, and even when miniature synaptic transmission was inhibited with botulinum neurotoxin C (BoNT/C). There were, however, marked differences in the morphology of individual spines induced by BDNF across these different levels of spontaneous ongoing synaptic activity. During both normal synaptic transmission, and when action potentials were blocked with TTX, BDNF increased the proportion of stubby, type-I spines. However, when SNARE-dependent vesicular release was inhibited with BoNT/C, BDNF increased the proportion of thin, type-III spines. Our results indicate that BDNF increases spine density irrespective of the levels of synaptic transmission. In addition, miniature synaptic transmission provides sufficient activity for the functional translation of BDNF-triggered spinogenesis into clearly defined morphological spine types, favouring those spines potentially responsible for coordinated Ca2+ transients thought to mediate synaptic plasticity. We propose that BDNF/TrkB signalling represents a mechanism of expression of both morphological and physiological homeostatic plasticity in the hippocampus, leading to a more efficient synaptic information transfer across widespread levels of synaptic activity.
Figures



Similar articles
-
BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses.J Neurosci. 2001 Jun 15;21(12):4249-58. doi: 10.1523/JNEUROSCI.21-12-04249.2001. J Neurosci. 2001. PMID: 11404410 Free PMC article.
-
Estradiol increases spine density and NMDA-dependent Ca2+ transients in spines of CA1 pyramidal neurons from hippocampal slices.J Neurophysiol. 1999 Mar;81(3):1404-11. doi: 10.1152/jn.1999.81.3.1404. J Neurophysiol. 1999. PMID: 10085365
-
ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons.Learn Mem. 2004 Mar-Apr;11(2):172-8. doi: 10.1101/lm.67804. Learn Mem. 2004. PMID: 15054132 Free PMC article.
-
Neurotrophins and hippocampal synaptic transmission and plasticity.J Neurosci Res. 1999 Oct 1;58(1):76-87. J Neurosci Res. 1999. PMID: 10491573 Review.
-
BDNF-induced local protein synthesis and synaptic plasticity.Neuropharmacology. 2014 Jan;76 Pt C:639-56. doi: 10.1016/j.neuropharm.2013.04.005. Epub 2013 Apr 16. Neuropharmacology. 2014. PMID: 23602987 Review.
Cited by
-
Brain-derived neurotrophic factor and epilepsy--a missing link?Epilepsy Curr. 2005 May-Jun;5(3):83-8. doi: 10.1111/j.1535-7511.2005.05312.x. Epilepsy Curr. 2005. PMID: 16145610 Free PMC article.
-
The potential neuroprotective effects of stingless bee honey.Front Aging Neurosci. 2023 Feb 8;14:1048028. doi: 10.3389/fnagi.2022.1048028. eCollection 2022. Front Aging Neurosci. 2023. PMID: 36846103 Free PMC article. Review.
-
NMDA receptor activation by spontaneous glutamatergic neurotransmission.J Neurophysiol. 2009 May;101(5):2290-6. doi: 10.1152/jn.90754.2008. Epub 2009 Mar 4. J Neurophysiol. 2009. PMID: 19261712 Free PMC article.
-
Hindered submicron mobility and long-term storage of presynaptic dense-core granules revealed by single-particle tracking.Dev Neurobiol. 2012 Sep;72(9):1181-95. doi: 10.1002/dneu.20984. Epub 2012 Jun 21. Dev Neurobiol. 2012. PMID: 21976424 Free PMC article.
-
BDNF repairs podocyte damage by microRNA-mediated increase of actin polymerization.J Pathol. 2015 Apr;235(5):731-44. doi: 10.1002/path.4484. Epub 2015 Jan 7. J Pathol. 2015. PMID: 25408545 Free PMC article.
References
-
- Bi G, Poo M. Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci. 2001;24:139–166. - PubMed
-
- Burrone J, O'Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. - PubMed
-
- Cabelli RJ, Shelton DL, Segal RA, Shatz CJ. Blockade of endogenous ligands of trkB inhibits formation of ocular dominance columns. Neuron. 1997;19:63–76. - PubMed
-
- Cajal SR. Histologie du Systeme Nerveux de l'Homme et des Vertebres. Vol. 1. Paris: 1909.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

