The GW182 protein colocalizes with mRNA degradation associated proteins hDcp1 and hLSm4 in cytoplasmic GW bodies
- PMID: 13130130
- PMCID: PMC1370480
- DOI: 10.1261/rna.5810203
The GW182 protein colocalizes with mRNA degradation associated proteins hDcp1 and hLSm4 in cytoplasmic GW bodies
Abstract
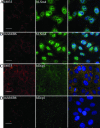
A novel cytoplasmic compartment referred to as GW bodies (GWBs) was initially identified using antibodies specific to a 182-kD protein termed GW182. GW182 was characterized by multiple glycine(G)-tryptophan(W) repeats and an RNA recognition motif (RRM) that bound a subset of HeLa cell messenger RNAs (mRNAs). The function of GWBs was not known; however, more recent evidence suggested similarities between GWBs and cytoplasmic structures that contain hLSm proteins and hDcp1, the human homolog to a yeast decapping enzyme subunit. In this study, we used antibodies to hLSm4 and hDcp1 to show that both of these markers of an mRNA degradation pathway colocalize to the same structures as GW182. Our studies demonstrate that GW182, hLSm4, and hDcp1 are found in the same cytoplasmic structures and suggest that GW182 is involved in the same mRNA processing pathway as hLSm4 and hDcp1.
Figures
Similar articles
-
A panel of monoclonal antibodies to cytoplasmic GW bodies and the mRNA binding protein GW182.Hybrid Hybridomics. 2003 Apr;22(2):79-86. doi: 10.1089/153685903321947996. Hybrid Hybridomics. 2003. PMID: 12831532
-
The human LSm1-7 proteins colocalize with the mRNA-degrading enzymes Dcp1/2 and Xrnl in distinct cytoplasmic foci.RNA. 2002 Dec;8(12):1489-501. RNA. 2002. PMID: 12515382 Free PMC article.
-
GW182 is critical for the stability of GW bodies expressed during the cell cycle and cell proliferation.J Cell Sci. 2004 Nov 1;117(Pt 23):5567-78. doi: 10.1242/jcs.01477. Epub 2004 Oct 19. J Cell Sci. 2004. PMID: 15494374
-
Function of GW182 and GW bodies in siRNA and miRNA pathways.Adv Exp Med Biol. 2013;768:71-96. doi: 10.1007/978-1-4614-5107-5_6. Adv Exp Med Biol. 2013. PMID: 23224966 Review.
-
Autoantibodies to protein transport and messenger RNA processing pathways: endosomes, lysosomes, Golgi complex, proteasomes, assemblyosomes, exosomes, and GW bodies.Clin Immunol. 2004 Jan;110(1):30-44. doi: 10.1016/j.clim.2003.10.005. Clin Immunol. 2004. PMID: 14962794 Review.
Cited by
-
P-body components LSM1, GW182, DDX3, DDX6 and XRN1 are recruited to WNV replication sites and positively regulate viral replication.Virology. 2013 Feb 5;436(1):1-7. doi: 10.1016/j.virol.2012.09.041. Epub 2012 Oct 24. Virology. 2013. PMID: 23102969 Free PMC article.
-
Deadenylation and P-bodies.Adv Exp Med Biol. 2013;768:183-95. doi: 10.1007/978-1-4614-5107-5_11. Adv Exp Med Biol. 2013. PMID: 23224971 Free PMC article. Review.
-
Formation of GW bodies is a consequence of microRNA genesis.EMBO Rep. 2006 Sep;7(9):904-10. doi: 10.1038/sj.embor.7400783. Epub 2006 Aug 11. EMBO Rep. 2006. PMID: 16906129 Free PMC article.
-
Posttranscriptional gene regulation by RNA-binding proteins during oxidative stress: implications for cellular senescence.Biol Chem. 2008 Mar;389(3):243-55. doi: 10.1515/BC.2008.022. Biol Chem. 2008. PMID: 18177264 Free PMC article. Review.
-
The fascinating world of RNA interference.Int J Biol Sci. 2009;5(2):97-117. doi: 10.7150/ijbs.5.97. Epub 2009 Jan 15. Int J Biol Sci. 2009. PMID: 19173032 Free PMC article. Review.
References
-
- Bollig, F., Winzen, R., Gaestel, M., Kostka, S., Resch, K., and Holtmann, H. 2003. Affinity purification of ARE-binding proteins identifies poly(A)-binding protein 1 as a potential substrate in MK2-induced mRNA stabilization. Biochem. Biophys. Res. Commun. 301: 665–670. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
