Cellular immunity elicited by human immunodeficiency virus type 1/ simian immunodeficiency virus DNA vaccination does not augment the sterile protection afforded by passive infusion of neutralizing antibodies
- PMID: 12970419
- PMCID: PMC228504
- DOI: 10.1128/jvi.77.19.10348-10356.2003
Cellular immunity elicited by human immunodeficiency virus type 1/ simian immunodeficiency virus DNA vaccination does not augment the sterile protection afforded by passive infusion of neutralizing antibodies
Abstract
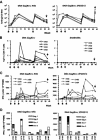
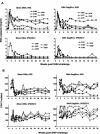
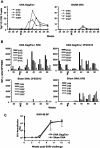
High levels of infused anti-human immunodeficiency virus type 1 (HIV-1) neutralizing monoclonal antibodies (MAbs) can completely protect macaque monkeys against mucosal chimeric simian-human immunodeficiency virus (SHIV) infection. Antibody levels below the protective threshold do not prevent infection but can substantially reduce plasma viremia. To assess if HIV-1/SIV-specific cellular immunity could combine with antibodies to produce sterile protection, we studied the effect of a suboptimal infusion of anti-HIV-1 neutralizing antibodies in macaques with active cellular immunity induced by interleukin-2 (IL-2)-adjuvanted DNA immunization. Twenty female macaques were divided into four groups: (i). DNA immunization plus irrelevant antibody, (ii). DNA immunization plus infusion of neutralizing MAbs 2F5 and 2G12, (iii). sham DNA plus 2F5 and 2G12, and (iv). sham DNA plus irrelevant antibody. DNA-immunized monkeys developed CD4 and CD8 T-cell responses as measured by epitope-specific tetramer staining and by pooled peptide ELISPOT assays for gamma interferon-secreting cells. After vaginal challenge, DNA-immunized animals that received irrelevant antibody became SHIV infected but displayed lower plasma viremia than control animals. Complete protection against SHIV challenge occurred in three animals that received sham DNA plus MAbs 2F5 and 2G12 and in two animals that received the DNA vaccine plus MAbs 2F5 and 2G12. Thus, although DNA immunization produced robust HIV-specific T-cell responses, we were unable to demonstrate that these responses contributed to the sterile protection mediated by passive infusion of neutralizing antibodies. These data suggest that although effector T cells can limit viral replication, they are not able to assist humoral immunity to prevent the establishment of initial infection.
Figures

Similar articles
-
Combination Adenovirus and Protein Vaccines Prevent Infection or Reduce Viral Burden after Heterologous Clade C Simian-Human Immunodeficiency Virus Mucosal Challenge.J Virol. 2018 Jan 2;92(2):e01092-17. doi: 10.1128/JVI.01092-17. Print 2018 Jan 15. J Virol. 2018. PMID: 29093095 Free PMC article.
-
Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies.Nat Med. 2000 Feb;6(2):207-10. doi: 10.1038/72318. Nat Med. 2000. PMID: 10655111
-
Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239.J Virol. 2002 Jul;76(14):7187-202. doi: 10.1128/jvi.76.14.7187-7202.2002. J Virol. 2002. PMID: 12072518 Free PMC article. Clinical Trial.
-
Antiviral CD8+ T cells in the genital tract control viral replication and delay progression to AIDS after vaginal SIV challenge in rhesus macaques immunized with virulence attenuated SHIV 89.6.J Intern Med. 2009 Jan;265(1):67-77. doi: 10.1111/j.1365-2796.2008.02051.x. J Intern Med. 2009. PMID: 19093961 Free PMC article. Review.
-
Protection of neonatal macaques against experimental SHIV infection by human neutralizing monoclonal antibodies.Transfus Clin Biol. 2001 Aug;8(4):350-8. doi: 10.1016/s1246-7820(01)00187-2. Transfus Clin Biol. 2001. PMID: 11642027 Review.
Cited by
-
Immunotherapies to prevent mother-to-child transmission of HIV.Curr HIV Res. 2013 Mar;11(2):137-43. doi: 10.2174/1570162x11311020006. Curr HIV Res. 2013. PMID: 23432489 Free PMC article. Review.
-
Good CoP, bad CoP? Interrogating the immune responses to primate lentiviral vaccines.Retrovirology. 2012 Oct 1;9:80. doi: 10.1186/1742-4690-9-80. Retrovirology. 2012. PMID: 23025660 Free PMC article. Review.
-
Induction of antibody-mediated neutralization in SIVmac239 by a naturally acquired V3 mutation.Virology. 2010 Apr 25;400(1):86-92. doi: 10.1016/j.virol.2010.01.014. Epub 2010 Feb 11. Virology. 2010. PMID: 20153009 Free PMC article.
-
Transfer of neutralizing IgG to macaques 6 h but not 24 h after SHIV infection confers sterilizing protection: implications for HIV-1 vaccine development.Proc Natl Acad Sci U S A. 2003 Dec 9;100(25):15131-6. doi: 10.1073/pnas.2436476100. Epub 2003 Nov 19. Proc Natl Acad Sci U S A. 2003. PMID: 14627745 Free PMC article.
-
Interactions between natural killer cells and antibody Fc result in enhanced antibody neutralization of human immunodeficiency virus type 1.J Virol. 2005 Feb;79(4):2042-9. doi: 10.1128/JVI.79.4.2042-2049.2005. J Virol. 2005. PMID: 15681406 Free PMC article.
References
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. - PubMed
-
- Armbruster, C., G. M. Stiegler, B. A. Vcelar, W. Jager, N. L. Michael, N. Vetter, and H. W. Katinger. 2002. A phase I trial with two human monoclonal antibodies (hMAb 2F5, 2G12) against HIV-1. AIDS 16:227-233. - PubMed
-
- Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. - PubMed
-
- Baig, J., D. B. Levy, P. F. McKay, J. E. Schmitz, S. Santra, R. A. Subbramanian, M. J. Kuroda, M. A. Lifton, D. A. Gorgone, L. S. Wyatt, B. Moss, Y. Huang, B. K. Chakrabarti, L. Xu, W. P. Kong, Z. Y. Yang, J. R. Mascola, G. J. Nabel, A. Carville, A. A. Lackner, R. S. Veazey, and N. L. Letvin. 2002. Elicitation of simian immunodeficiency virus-specific cytotoxic T lymphocytes in mucosal compartments of rhesus monkeys by systemic vaccination. J. Virol. 76:11484-11490. - PMC - PubMed
-
- Barouch, D. H., A. Craiu, M. J. Kuroda, J. E. Schmitz, X. X. Zheng, S. Santra, J. D. Frost, G. R. Krivulka, M. A. Lifton, C. L. Crabbs, G. Heidecker, H. C. Perry, M. E. Davies, H. Xie, C. E. Nickerson, T. D. Steenbeke, C. I. Lord, D. C. Montefiori, T. B. Strom, J. W. Shiver, M. G. Lewis, and N. L. Letvin. 2000. Augmentation of immune responses to HIV-1 and simian immunodeficiency virus DNA vaccines by IL-2/Ig plasmid administration in rhesus monkeys. Proc. Natl. Acad. Sci. USA 97:4192-4197. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

