Herpesvirus entry: an update
- PMID: 12970403
- PMCID: PMC228481
- DOI: 10.1128/jvi.77.19.10179-10185.2003
Herpesvirus entry: an update
Figures
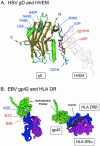
Similar articles
-
Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry.J Clin Invest. 2001 Aug;108(4):503-10. doi: 10.1172/JCI13799. J Clin Invest. 2001. PMID: 11518721 Free PMC article. Review. No abstract available.
-
gH/gL supercomplexes at early stages of herpesvirus entry.Curr Opin Virol. 2016 Jun;18:1-8. doi: 10.1016/j.coviro.2016.01.010. Epub 2016 Feb 2. Curr Opin Virol. 2016. PMID: 26849495 Free PMC article. Review.
-
Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry.EMBO J. 2005 Dec 7;24(23):4144-53. doi: 10.1038/sj.emboj.7600875. Epub 2005 Nov 17. EMBO J. 2005. PMID: 16292345 Free PMC article.
-
Herpesvirus lytic replication and the cell cycle: arresting new developments.J Virol. 2001 May;75(10):4475-81. doi: 10.1128/JVI.75.10.4475-4481.2001. J Virol. 2001. PMID: 11312317 Free PMC article. Review. No abstract available.
-
[Mechanisms of herpesvirus infection--virus entry into host cells and virus assembly].Uirusu. 2007 Dec;57(2):151-8. doi: 10.2222/jsv.57.151. Uirusu. 2007. PMID: 18357753 Review. Japanese.
Cited by
-
Role of Filopodia in HSV-1 Entry into Zebrafish 3-O-Sulfotransferase-3-Expressing Cells.Open Virol J. 2013 Apr 5;7:41-8. doi: 10.2174/1874357901307010041. Print 2013. Open Virol J. 2013. PMID: 23667409 Free PMC article.
-
KSHV (HHV8) vaccine: promises and potential pitfalls for a new anti-cancer vaccine.NPJ Vaccines. 2022 Sep 20;7(1):108. doi: 10.1038/s41541-022-00535-4. NPJ Vaccines. 2022. PMID: 36127367 Free PMC article. Review.
-
Herpes simplex virus 1 glycoprotein M and the membrane-associated protein UL11 are required for virus-induced cell fusion and efficient virus entry.J Virol. 2013 Jul;87(14):8029-37. doi: 10.1128/JVI.01181-13. Epub 2013 May 15. J Virol. 2013. PMID: 23678175 Free PMC article.
-
KEGG orthology-based annotation of the predicted proteome of Acropora digitifera: ZoophyteBase - an open access and searchable database of a coral genome.BMC Genomics. 2013 Jul 26;14:509. doi: 10.1186/1471-2164-14-509. BMC Genomics. 2013. PMID: 23889801 Free PMC article.
-
The precise function of alphaherpesvirus tegument proteins and their interactions during the viral life cycle.Front Microbiol. 2024 Jul 2;15:1431672. doi: 10.3389/fmicb.2024.1431672. eCollection 2024. Front Microbiol. 2024. PMID: 39015737 Free PMC article. Review.
References
-
- Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2001. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284:235-249. - PubMed
-
- Akula, S. M., N. P. Pramod, F.-Z. Wang, and B. Chandran. 2002. Integrin α3β1 (CD49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. - PubMed
-
- Borza, C. M., and L. M. Hutt-Fletcher. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 8:594-599. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA21776/CA/NCI NIH HHS/United States
- R01 CA093444/CA/NCI NIH HHS/United States
- R01 AI049394/AI/NIAID NIH HHS/United States
- AI36293/AI/NIAID NIH HHS/United States
- AI53774/AI/NIAID NIH HHS/United States
- R01 CA062234/CA/NCI NIH HHS/United States
- R01 DE013127/DE/NIDCR NIH HHS/United States
- R37 AI036293/AI/NIAID NIH HHS/United States
- AI49394/AI/NIAID NIH HHS/United States
- CA62234/CA/NCI NIH HHS/United States
- CA93444/CA/NCI NIH HHS/United States
- R01 CA073507/CA/NCI NIH HHS/United States
- AI31494/AI/NIAID NIH HHS/United States
- CA73507/CA/NCI NIH HHS/United States
- U19 AI031494/AI/NIAID NIH HHS/United States
- R01 CA021776/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

