Genetic and cellular basis for acetylcholine inhibition of Caenorhabditis elegans egg-laying behavior
- PMID: 12954868
- PMCID: PMC6740490
- DOI: 10.1523/JNEUROSCI.23-22-08060.2003
Genetic and cellular basis for acetylcholine inhibition of Caenorhabditis elegans egg-laying behavior
Abstract
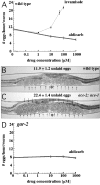
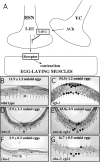
Egg-laying behavior in Caenorhabditis elegans is activated by signaling through the G-protein G(rho)q and inhibited by signaling through a second G-protein, G(rho)o. Activation of egg laying depends on the serotonergic hermaphrodite-specific neurons (HSNs), but the neurotransmitter(s) and cell(s) that signal to inhibit egg laying are not known. Mutants for G-protein signaling genes have well characterized defects in egg laying. Here we present an analysis of mutants for other genes reported to lack inhibition of egg laying. Of the nine strongest, six have morphological defects in the ventral-type C (VC) neurons, which synapse onto both the HSNs and the egg-laying muscles and are thus the third cell type comprising the egg-laying system. Laser-ablating VC neurons could also disrupt the inhibition of egg laying. The remaining three mutants (unc-4, cha-1, and unc-17) are defective for synthesis or packaging of acetylcholine in the VCs. The egg-laying defects of unc-4, cha-1, and unc-17 were rescued by VC-specific expression of the corresponding cDNAs. In addition, increasing synaptic acetylcholine by reducing acetylcholinesterase activity, with either mutations or the inhibitor aldicarb, decreased egg laying. Finally, we found that a knock-out for the HSN-expressed receptor G-protein-coupled acetylcholine receptor 2 (GAR-2) shows a partial defect in the inhibition of egg laying and fails to respond to aldicarb. Our results show that acetylcholine released from the VC neurons inhibits egg-laying behavior. This inhibition may be caused, in part, by acetylcholine signaling onto the HSN presynaptic terminals, via GAR-2, to inhibit neurotransmitter release.
Figures
Similar articles
-
Activation of EGL-47, a Galpha(o)-coupled receptor, inhibits function of hermaphrodite-specific motor neurons to regulate Caenorhabditis elegans egg-laying behavior.J Neurosci. 2004 Sep 29;24(39):8522-30. doi: 10.1523/JNEUROSCI.1915-04.2004. J Neurosci. 2004. PMID: 15456826 Free PMC article.
-
Serotonin and neuropeptides are both released by the HSN command neuron to initiate Caenorhabditis elegans egg laying.PLoS Genet. 2019 Jan 24;15(1):e1007896. doi: 10.1371/journal.pgen.1007896. eCollection 2019 Jan. PLoS Genet. 2019. PMID: 30677018 Free PMC article.
-
Egg-laying defective mutants of the nematode Caenorhabditis elegans.Genetics. 1983 Aug;104(4):619-47. doi: 10.1093/genetics/104.4.619. Genetics. 1983. PMID: 11813735 Free PMC article.
-
Acetylcholine.WormBook. 2007 Jan 30:1-21. doi: 10.1895/wormbook.1.131.1. WormBook. 2007. PMID: 18050502 Free PMC article. Review.
-
Genetics of egg-laying in worms.Annu Rev Genet. 2006;40:487-509. doi: 10.1146/annurev.genet.40.110405.090527. Annu Rev Genet. 2006. PMID: 17094742 Review.
Cited by
-
ACR-12 ionotropic acetylcholine receptor complexes regulate inhibitory motor neuron activity in Caenorhabditis elegans.J Neurosci. 2013 Mar 27;33(13):5524-32. doi: 10.1523/JNEUROSCI.4384-12.2013. J Neurosci. 2013. PMID: 23536067 Free PMC article.
-
The RHO-1 RhoGTPase modulates fertility and multiple behaviors in adult C. elegans.PLoS One. 2011 Feb 28;6(2):e17265. doi: 10.1371/journal.pone.0017265. PLoS One. 2011. PMID: 21387015 Free PMC article.
-
Identification of an Ascaris G protein-coupled acetylcholine receptor with atypical muscarinic pharmacology.Int J Parasitol. 2009 Sep;39(11):1215-22. doi: 10.1016/j.ijpara.2009.03.001. Epub 2009 Mar 25. Int J Parasitol. 2009. PMID: 19327362 Free PMC article.
-
The Annona muricata leaf ethanol extract affects mobility and reproduction in mutant strain NB327 Caenorhabditis elegans.Biochem Biophys Rep. 2017 Apr 24;10:282-286. doi: 10.1016/j.bbrep.2017.04.016. eCollection 2017 Jul. Biochem Biophys Rep. 2017. PMID: 28955756 Free PMC article.
-
Highly sensitive isotope-dilution liquid-chromatography-electrospray ionization-tandem-mass spectrometry approach to study the drug-mediated modulation of dopamine and serotonin levels in Caenorhabditis elegans.Talanta. 2015 Nov 1;144:71-9. doi: 10.1016/j.talanta.2015.05.057. Epub 2015 May 28. Talanta. 2015. PMID: 26452793 Free PMC article.
References
-
- Alfonso A, Grundahl K, Duerr JS, Han HP, Rand JB ( 1993) The Caenorhabditis elegans unc-17 gene: a putative vesicular acetylcholine transporter. Science 261: 617-619. - PubMed
-
- Baran R, Aronoff R, Garriga G ( 1999) The C. elegans homeodomain gene unc-42 regulates chemosensory and glutamate receptor expression. Development 126: 2241-2251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
