DNA condensation by the nucleocapsid protein of HIV-1: a mechanism ensuring DNA protection
- PMID: 12954779
- PMCID: PMC203321
- DOI: 10.1093/nar/gkg738
DNA condensation by the nucleocapsid protein of HIV-1: a mechanism ensuring DNA protection
Abstract
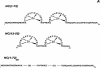
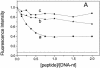
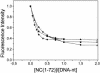
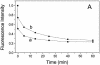
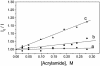
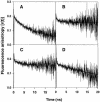
The nucleocapsid (NC) protein NCp7 of the immunodeficiency virus type 1 is a small basic protein with two zinc finger motifs. NCp7 has key roles in virus replication and structure, which rely on its interactions with nucleic acids. Although most interactions involve RNAs, binding to the viral DNA is thought to be of importance to achieve protection of the DNA against cellular nucleases and its integration into the host genome. We investigated the interaction of NCp7 with plasmid DNA as a model system. The fluorescence probe YOYO-1 was used as the reporter. Binding of NCp7 to DNA caused DNA condensation, as inferred from the dramatic decrease in YOYO-1 fluorescence. Efficient condensation of DNA required the full length NCp7 with the zinc fingers. The fingerless peptide was less efficient in condensing DNA. Binding of both these NC peptides led to freezing of the segmental dynamics of DNA as revealed by anisotropy decay kinetics of YOYO-1. The truncated peptide NC(12-55) which retains the zinc fingers did not lead to DNA condensation despite its ability to bind and partially freeze the segmental motion of DNA. We propose that the histone-like property of NCp7 leading to DNA condensation contributes to viral DNA stability, in vivo.
Figures



Similar articles
-
HIV-1 nucleocapsid protein activates transient melting of least stable parts of the secondary structure of TAR and its complementary sequence.J Mol Biol. 2002 Mar 29;317(3):385-99. doi: 10.1006/jmbi.2002.5429. J Mol Biol. 2002. PMID: 11922672
-
Analysis of NCp7-dependent activation of HIV-1 cDNA integration and its conservation among retroviral nucleocapsid proteins.J Mol Biol. 2003 Jun 6;329(3):411-21. doi: 10.1016/s0022-2836(03)00472-8. J Mol Biol. 2003. PMID: 12767826
-
Effects of temperature on the dynamic behaviour of the HIV-1 nucleocapsid NCp7 and its DNA complex.J Mol Biol. 2002 Feb 22;316(3):611-27. doi: 10.1006/jmbi.2001.5379. J Mol Biol. 2002. PMID: 11866521
-
Structure, biological functions and inhibition of the HIV-1 proteins Vpr and NCp7.Biochimie. 1997 Nov;79(11):673-80. doi: 10.1016/s0300-9084(97)83501-8. Biochimie. 1997. PMID: 9479450 Review.
-
The chaperoning and assistance roles of the HIV-1 nucleocapsid protein in proviral DNA synthesis and maintenance.Curr HIV Res. 2004 Jan;2(1):79-92. doi: 10.2174/1570162043485022. Curr HIV Res. 2004. PMID: 15053342 Review.
Cited by
-
Single-molecule spectroscopic study of dynamic nanoscale DNA bending behavior of HIV-1 nucleocapsid protein.J Phys Chem B. 2013 Apr 25;117(16):4183-96. doi: 10.1021/jp3018259. Epub 2012 May 16. J Phys Chem B. 2013. PMID: 22591315 Free PMC article.
-
Histones Are Rapidly Loaded onto Unintegrated Retroviral DNAs Soon after Nuclear Entry.Cell Host Microbe. 2016 Dec 14;20(6):798-809. doi: 10.1016/j.chom.2016.10.009. Epub 2016 Nov 17. Cell Host Microbe. 2016. PMID: 27866901 Free PMC article.
-
Synthesis of distal and proximal fleximer base analogues and evaluation in the nucleocapsid protein of HIV-1.Bioorg Med Chem. 2019 Jul 1;27(13):2883-2892. doi: 10.1016/j.bmc.2019.05.019. Epub 2019 May 15. Bioorg Med Chem. 2019. PMID: 31126822 Free PMC article.
-
Nucleocapsid protein function in early infection processes.Virus Res. 2008 Jun;134(1-2):39-63. doi: 10.1016/j.virusres.2007.12.006. Epub 2008 Feb 14. Virus Res. 2008. PMID: 18279991 Free PMC article. Review.
-
DNA binding and condensation properties of the herpes simplex virus type 1 triplex protein VP19C.PLoS One. 2014 Aug 14;9(8):e104640. doi: 10.1371/journal.pone.0104640. eCollection 2014. PLoS One. 2014. PMID: 25121591 Free PMC article.
References
-
- Mély Y., De Rocquigny,H., Morellet,N., Roques,B.P. and Gérard,D. (1996) Zinc binding to the HIV-1 nucleocapsid protein: a thermodynamic investigation by fluorescence spectroscopy. Biochemistry, 35, 5175–5182. - PubMed
-
- Darlix J.L., Lapadat-Tapolsky,M., de Rocquigny,H. and Roques,B.P. (1995) First glimpses at structure-function relationships of the nucleocapsid protein of retroviruses. J. Mol. Biol., 254, 523–537. - PubMed
-
- Coffin J.M. (1984) Structure of the retroviral genome. In Weiss,R., Teich,N., Varmus,H. and Coffin,J. (eds), RNA Tumor Viruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 261–368.
-
- Tanchou V., Gabus,C., Rogemond,V. and Darlix,J.L. (1995) Formation of stable and functional HIV-1 nucleoprotein complexes in vitro. J. Mol. Biol., 252, 563–571. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

