Dynamic phosphoregulation of the cortical actin cytoskeleton and endocytic machinery revealed by real-time chemical genetic analysis
- PMID: 12952930
- PMCID: PMC2172809
- DOI: 10.1083/jcb.200305077
Dynamic phosphoregulation of the cortical actin cytoskeleton and endocytic machinery revealed by real-time chemical genetic analysis
Abstract
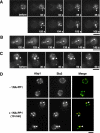
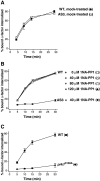
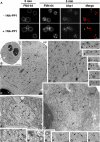
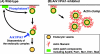
We used chemical genetics to control the activity of budding yeast Prk1p, which is a protein kinase that is related to mammalian GAK and AAK1, and which targets several actin regulatory proteins implicated in endocytosis. In vivo Prk1p inhibition blocked pheromone receptor endocytosis, and caused cortical actin patches to rapidly aggregate into large clumps that contained Abp1p, Sla2p, Pan1p, Sla1p, and Ent1p. Clump formation depended on Arp2p, suggesting that this phenotype might result from unregulated Arp2/3-stimulated actin assembly. Electron microscopy/immunoelectron microscopy analysis and tracking of the endocytic membrane marker FM4-64 revealed vesicles of likely endocytic origin within the actin clumps. Upon inhibitor washout, the actin clumps rapidly disassembled, and properly polarized actin patches reappeared. Our results suggest that actin clumps result from blockage at a normally transient step during which actin assembly is stimulated by endocytic proteins. Thus, we revealed tight phosphoregulation of an intrinsically dynamic, actin patch-related process, and propose that Prk1p negatively regulates the actin assembly-stimulating activity of endocytic proteins.
Figures


Similar articles
-
In vivo role for actin-regulating kinases in endocytosis and yeast epsin phosphorylation.Mol Biol Cell. 2001 Nov;12(11):3668-79. doi: 10.1091/mbc.12.11.3668. Mol Biol Cell. 2001. PMID: 11694597 Free PMC article.
-
Regulation of yeast actin cytoskeleton-regulatory complex Pan1p/Sla1p/End3p by serine/threonine kinase Prk1p.Mol Biol Cell. 2001 Dec;12(12):3759-72. doi: 10.1091/mbc.12.12.3759. Mol Biol Cell. 2001. PMID: 11739778 Free PMC article.
-
Novel protein kinases Ark1p and Prk1p associate with and regulate the cortical actin cytoskeleton in budding yeast.J Cell Biol. 1999 Mar 22;144(6):1203-18. doi: 10.1083/jcb.144.6.1203. J Cell Biol. 1999. PMID: 10087264 Free PMC article.
-
Pan1p: an actin director of endocytosis in yeast.Int J Biochem Cell Biol. 2007;39(10):1760-4. doi: 10.1016/j.biocel.2006.12.001. Epub 2006 Dec 21. Int J Biochem Cell Biol. 2007. PMID: 17303466 Review.
-
Prk1p.Int J Biochem Cell Biol. 2005 Jan;37(1):48-53. doi: 10.1016/j.biocel.2004.03.010. Int J Biochem Cell Biol. 2005. PMID: 15381149 Review.
Cited by
-
Defects in structural integrity of ergosterol and the Cdc50p-Drs2p putative phospholipid translocase cause accumulation of endocytic membranes, onto which actin patches are assembled in yeast.Mol Biol Cell. 2005 Dec;16(12):5592-609. doi: 10.1091/mbc.e05-05-0452. Epub 2005 Sep 29. Mol Biol Cell. 2005. PMID: 16195350 Free PMC article.
-
Clathrin-mediated endocytosis in budding yeast.Trends Cell Biol. 2012 Jan;22(1):1-13. doi: 10.1016/j.tcb.2011.09.001. Epub 2011 Oct 20. Trends Cell Biol. 2012. PMID: 22018597 Free PMC article. Review.
-
Sequential counteracting kinases restrict an asymmetric gene expression program to early G1.Mol Biol Cell. 2010 Aug 15;21(16):2809-20. doi: 10.1091/mbc.E10-02-0174. Epub 2010 Jun 23. Mol Biol Cell. 2010. PMID: 20573982 Free PMC article.
-
Conserved Ark1-related kinases function in a TORC2 signaling network.Mol Biol Cell. 2020 Aug 15;31(18):2057-2069. doi: 10.1091/mbc.E19-12-0685. Epub 2020 Jul 2. Mol Biol Cell. 2020. PMID: 32614710 Free PMC article.
-
Phosphoproteomics Meets Chemical Genetics: Approaches for Global Mapping and Deciphering the Phosphoproteome.Int J Mol Sci. 2020 Oct 15;21(20):7637. doi: 10.3390/ijms21207637. Int J Mol Sci. 2020. PMID: 33076458 Free PMC article. Review.
References
-
- Ayscough, K.R., J. Stryker, N. Pokala, M. Sanders, P. Crews, and D.G. Drubin. 1997. High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 137:399–416. - PMC - PubMed
-
- Bishop, A.C., K. Shah, Y. Liu, L. Witucki, C. Kung, and K.M. Shokat. 1998. Design of allele-specific inhibitors to probe protein kinase signaling. Curr. Biol. 8:257–266. - PubMed
-
- Bishop, A.C., O. Buzko, and K.M. Shokat. 2001. Magic bullets for protein kinases. 2001. Trends Cell Biol. 11:167–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

