Maedi-visna virus and caprine arthritis encephalitis virus genomes encode a Vpr-like but no Tat protein
- PMID: 12915575
- PMCID: PMC187391
- DOI: 10.1128/jvi.77.17.9632-9638.2003
Maedi-visna virus and caprine arthritis encephalitis virus genomes encode a Vpr-like but no Tat protein
Abstract
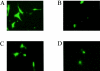
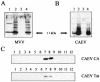
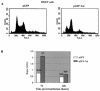
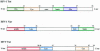
A small open reading frame (ORF) in maedi-visna virus (MVV) and caprine arthritis encephalitis virus (CAEV) was initially named "tat" by analogy with a similarly placed ORF in the primate lentiviruses. The encoded "Tat" protein was ascribed the function of up regulation of the viral transcription from the long terminal repeat (LTR) promoter, but we have recently reported that MVV and CAEV Tat proteins lack trans-activation function activity under physiological conditions (S. Villet, C. Faure, B. Bouzar, G. Verdien, Y. Chebloune, and C. Legras, Virology 307:317-327, 2003). In the present work, we show that MVV Tat localizes to the nucleus of transfected cells, probably through the action of a nuclear localization signal in its C-terminal portion. We also show that, unlike the human immunodeficiency virus (HIV) Tat protein, MVV Tat was not secreted into the medium by transfected human or caprine cells in the absence of cell lysis but that, like the primate accessory protein Vpr, MVV and CAEV Tat proteins were incorporated into viral particles. In addition, analysis of the primary protein structures showed that small-ruminant lentivirus (SRLV) Tat proteins are more similar to the HIV type 1 (HIV-1) Vpr protein than to HIV-1 Tat. We also demonstrate a functional similarity between the SRLV Tat proteins and the HIV-1 Vpr product in the induction of a specific G(2) arrest of the cell cycle in MVV Tat-transfected cells, which increases the G(2)/G(1) ratio 2.8-fold. Together, these data strongly suggest that the tat ORF in the SRLV genomes does not code for a regulatory transactivator of the LTR but, rather, for a Vpr-like accessory protein.
Figures

Similar articles
-
Lack of trans-activation function for Maedi Visna virus and Caprine arthritis encephalitis virus Tat proteins.Virology. 2003 Mar 15;307(2):317-27. doi: 10.1016/s0042-6822(02)00076-4. Virology. 2003. PMID: 12667801
-
Specific G2 arrest of caprine cells infected with a caprine arthritis encephalitis virus expressing vpr and vpx genes from simian immunodeficiency virus.Virology. 2003 Apr 25;309(1):41-52. doi: 10.1016/s0042-6822(03)00014-x. Virology. 2003. PMID: 12726725
-
The CAEV tat gene trans-activates the viral LTR and is necessary for efficient viral replication.Virology. 1993 Nov;197(1):35-44. doi: 10.1006/viro.1993.1564. Virology. 1993. PMID: 8212571
-
Maedi-visna virus and its relationship to human immunodeficiency virus.AIDS Rev. 2005 Oct-Dec;7(4):233-45. AIDS Rev. 2005. PMID: 16425963 Review.
-
Small ruminant lentiviruses: immunopathogenesis of visna-maedi and caprine arthritis and encephalitis virus.Comp Immunol Microbiol Infect Dis. 2012 May;35(3):259-69. doi: 10.1016/j.cimid.2011.12.003. Epub 2012 Jan 9. Comp Immunol Microbiol Infect Dis. 2012. PMID: 22237012 Review.
Cited by
-
Non-Primate Lentiviral Vectors and Their Applications in Gene Therapy for Ocular Disorders.Viruses. 2018 Jun 9;10(6):316. doi: 10.3390/v10060316. Viruses. 2018. PMID: 29890733 Free PMC article. Review.
-
Gene Therapy Applications of Non-Human Lentiviral Vectors.Viruses. 2020 Sep 29;12(10):1106. doi: 10.3390/v12101106. Viruses. 2020. PMID: 33003635 Free PMC article. Review.
-
Duplicated sequence motif in the long terminal repeat of maedi-visna virus extends cell tropism and is associated with neurovirulence.J Virol. 2007 Apr;81(8):4052-7. doi: 10.1128/JVI.02319-06. Epub 2007 Feb 7. J Virol. 2007. PMID: 17287273 Free PMC article.
-
Long-term central and effector SHIV-specific memory T cell responses elicited after a single immunization with a novel lentivector DNA vaccine.PLoS One. 2014 Oct 22;9(10):e110883. doi: 10.1371/journal.pone.0110883. eCollection 2014. PLoS One. 2014. PMID: 25337803 Free PMC article.
-
Characterization of small ruminant lentivirus A4 subtype isolates and assessment of their pathogenic potential in naturally infected goats.Virol J. 2014 Apr 3;11:65. doi: 10.1186/1743-422X-11-65. Virol J. 2014. PMID: 24708706 Free PMC article.
References
-
- Andresson, O. S., J. E. Elser, G. J. Tobin, J. D. Greenwood, M. A. Gonda, G. Georgsson, V. Andresdottir, E. Benediktsdottir, H. M. Carlsdottir, and E. O. Mantyla. 1993. Nucleotide sequence and biological properties of a pathogenic proviral molecular clone of neurovirulent visna virus. Virology 193:89-105. - PubMed
-
- Cork, L. C., W. J. Hadlow, T. B. Crawford, J. R. Gorham, and R. C. Piper. 1974. Infectious leukoencephalomyelitis of young goats J. Infect. Dis. 129:134-141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

