After Hrs with HIV
- PMID: 12900390
- PMCID: PMC2172700
- DOI: 10.1083/jcb.200307062
After Hrs with HIV
Abstract
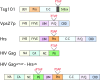
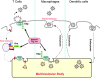
To efficiently bud off from infected cells, HIV and other enveloped viruses hijack the host cellular machinery that is normally involved in vacuolar protein sorting and multivesicular body (MVB) biogenesis. The HIV Gag protein mimics hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs), a modular adaptor protein that links membrane cargo recognition to its degradation after delivery to MVBs. In contrast to T cells, where HIV budding occurs at the plasma membrane, virus buds into vacuoles of macrophages, a process that may facilitate its spread within the infected host.
Figures
Similar articles
-
HIV Gag mimics the Tsg101-recruiting activity of the human Hrs protein.J Cell Biol. 2003 Aug 4;162(3):425-34. doi: 10.1083/jcb.200302138. J Cell Biol. 2003. PMID: 12900394 Free PMC article.
-
Infectious HIV-1 assembles in late endosomes in primary macrophages.J Cell Biol. 2003 Aug 4;162(3):443-55. doi: 10.1083/jcb.200304008. Epub 2003 Jul 28. J Cell Biol. 2003. PMID: 12885763 Free PMC article.
-
[HIV budding and Tsg101].Uirusu. 2005 Dec;55(2):281-6. doi: 10.2222/jsv.55.281. Uirusu. 2005. PMID: 16557014 Review. Japanese.
-
Inhibition or deficiency of cathepsin B leads defects in HIV-1 Gag pseudoparticle release in macrophages and HEK293T cells.Antiviral Res. 2012 Jan;93(1):175-84. doi: 10.1016/j.antiviral.2011.11.009. Epub 2011 Nov 26. Antiviral Res. 2012. PMID: 22138708
-
Protein sorting into multivesicular endosomes.Curr Opin Cell Biol. 2003 Aug;15(4):446-55. doi: 10.1016/s0955-0674(03)00080-2. Curr Opin Cell Biol. 2003. PMID: 12892785 Review.
Cited by
-
Biochemical and biologic characterization of exosomes and microvesicles as facilitators of HIV-1 infection in macrophages.J Immunol. 2012 Jul 15;189(2):744-54. doi: 10.4049/jimmunol.1102244. Epub 2012 Jun 18. J Immunol. 2012. PMID: 22711894 Free PMC article.
-
S protein of severe acute respiratory syndrome-associated coronavirus mediates entry into hepatoma cell lines and is targeted by neutralizing antibodies in infected patients.J Virol. 2004 Jun;78(12):6134-42. doi: 10.1128/JVI.78.12.6134-6142.2004. J Virol. 2004. PMID: 15163706 Free PMC article.
-
Intracellular assembly and budding of the Murine Leukemia Virus in infected cells.Retrovirology. 2006 Feb 10;3:12. doi: 10.1186/1742-4690-3-12. Retrovirology. 2006. PMID: 16472393 Free PMC article.
-
Early steps of retrovirus replicative cycle.Retrovirology. 2004 May 14;1:9. doi: 10.1186/1742-4690-1-9. Retrovirology. 2004. PMID: 15169567 Free PMC article. Review.
-
The cellular HIV-1 Rev cofactor hRIP is required for viral replication.Proc Natl Acad Sci U S A. 2005 Mar 15;102(11):4027-32. doi: 10.1073/pnas.0408889102. Epub 2005 Mar 4. Proc Natl Acad Sci U S A. 2005. PMID: 15749819 Free PMC article.
References
-
- Babst, M., D.J. Katzmann, E.J. Estepa-Sabal, T. Meerloo, and S.D. Emr. 2002. a. ESCRT-III: an endosome-associated heterooligomeric protein complex required for MVB sorting. Dev. Cell. 3:271–282. - PubMed
-
- Babst, M., D.J. Katzmann, W.B. Snyder, B. Wendland, and S.D. Emr. 2002. b. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell. 3:283–289. - PubMed
-
- Bilodeau, P.S., J.L. Urbanowski, S.C. Winistorfer, and R.C. Piper. 2002. The Vps27p Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nat. Cell Biol. 4:534–539. - PubMed
-
- Clague, M.J. 2002. Membrane transport: a coat for ubiquitin. Curr. Biol. 12:R529–R531. - PubMed

