Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors
- PMID: 12881415
- PMCID: PMC169039
- DOI: 10.1093/emboj/cdg366
Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors
Abstract
Traditional treatment of infectious diseases is based on compounds that kill or inhibit growth of bacteria. A major concern with this approach is the frequent development of resistance to antibiotics. The discovery of communication systems (quorum sensing systems) regulating bacterial virulence has afforded a novel opportunity to control infectious bacteria without interfering with growth. Compounds that can override communication signals have been found in the marine environment. Using Pseudomonas aeruginosa PAO1 as an example of an opportunistic human pathogen, we show that a synthetic derivate of natural furanone compounds can act as a potent antagonist of bacterial quorum sensing. We employed GeneChip microarray technology to identify furanone target genes and to map the quorum sensing regulon. The transcriptome analysis showed that the furanone drug specifically targeted quorum sensing systems and inhibited virulence factor expression. Application of the drug to P.aeruginosa biofilms increased bacterial susceptibility to tobramycin and SDS. In a mouse pulmonary infection model, the drug inhibited quorum sensing of the infecting bacteria and promoted their clearance by the mouse immune response.
Figures

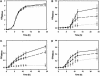






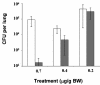
Similar articles
-
Synthetic furanones inhibit quorum-sensing and enhance bacterial clearance in Pseudomonas aeruginosa lung infection in mice.J Antimicrob Chemother. 2004 Jun;53(6):1054-61. doi: 10.1093/jac/dkh223. Epub 2004 Apr 29. J Antimicrob Chemother. 2004. PMID: 15117922
-
Bacteria that inhibit quorum sensing decrease biofilm formation and virulence in Pseudomonas aeruginosa PAO1.Pathog Dis. 2014 Apr;70(3):271-9. doi: 10.1111/2049-632X.12124. Epub 2014 Jan 27. Pathog Dis. 2014. PMID: 24415453
-
Association of furanone C-30 with biofilm formation & antibiotic resistance in Pseudomonas aeruginosa.Indian J Med Res. 2018 Apr;147(4):400-406. doi: 10.4103/ijmr.IJMR_2010_16. Indian J Med Res. 2018. PMID: 29998876 Free PMC article.
-
[Quorum sensing: a new clinical target for Pseudomonas aeruginosa?].Med Mal Infect. 2006 Jul;36(7):349-57. doi: 10.1016/j.medmal.2006.01.008. Epub 2006 May 2. Med Mal Infect. 2006. PMID: 16631332 Review. French.
-
Targeting quorum sensing in Pseudomonas aeruginosa biofilms: current and emerging inhibitors.Future Microbiol. 2013 Jul;8(7):901-21. doi: 10.2217/fmb.13.57. Future Microbiol. 2013. PMID: 23841636 Review.
Cited by
-
Andrographis paniculata (Burm. f.) Wall. ex Nees: An Updated Review of Phytochemistry, Antimicrobial Pharmacology, and Clinical Safety and Efficacy.Life (Basel). 2021 Apr 16;11(4):348. doi: 10.3390/life11040348. Life (Basel). 2021. PMID: 33923529 Free PMC article. Review.
-
New life for an old drug: the anthelmintic drug niclosamide inhibits Pseudomonas aeruginosa quorum sensing.Antimicrob Agents Chemother. 2013 Feb;57(2):996-1005. doi: 10.1128/AAC.01952-12. Epub 2012 Dec 17. Antimicrob Agents Chemother. 2013. PMID: 23254430 Free PMC article.
-
Antibiofilm and Antivirulence Activities of Gold and Zinc Oxide Nanoparticles Synthesized from Kimchi-Isolated Leuconostoc sp. Strain C2.Antibiotics (Basel). 2022 Nov 1;11(11):1524. doi: 10.3390/antibiotics11111524. Antibiotics (Basel). 2022. PMID: 36358180 Free PMC article.
-
Paraoxonases-2 and -3 Are Important Defense Enzymes against Pseudomonas aeruginosa Virulence Factors due to Their Anti-Oxidative and Anti-Inflammatory Properties.J Lipids. 2012;2012:352857. doi: 10.1155/2012/352857. Epub 2012 Apr 12. J Lipids. 2012. PMID: 22570791 Free PMC article.
-
Inhibition of Quorum-Sensing Regulator from Pseudomonas aeruginosa Using a Flavone Derivative.Molecules. 2022 Apr 10;27(8):2439. doi: 10.3390/molecules27082439. Molecules. 2022. PMID: 35458637 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

