Assessment of the relative contribution of COX-1 and COX-2 isoforms to ischemia-induced oxidative damage and neurodegeneration following transient global cerebral ischemia
- PMID: 12859668
- PMCID: PMC1636020
- DOI: 10.1046/j.1471-4159.2003.01812.x
Assessment of the relative contribution of COX-1 and COX-2 isoforms to ischemia-induced oxidative damage and neurodegeneration following transient global cerebral ischemia
Abstract
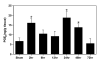
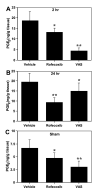
We investigated the relative contribution of COX-1 and/or COX-2 to oxidative damage, prostaglandin E2 (PGE2) production and hippocampal CA1 neuronal loss in a model of 5 min transient global cerebral ischemia in gerbils. Our results revealed a biphasic and significant increase in PGE2 levels after 2 and 24-48 h of reperfusion. The late increase in PGE2 levels (24 h) was more potently reduced by the highly selective COX-2 inhibitor rofecoxib (20 mg/kg) relative to the COX-1 inhibitor valeryl salicylate (20 mg/kg). The delayed rise in COX catalytic activity preceded the onset of histopathological changes in the CA1 subfield of the hippocampus. Post-ischemia treatment with rofecoxib (starting 6 h after restoration of blood flow) significantly reduced measures of oxidative damage (glutathione depletion and lipid peroxidation) seen at 48 h after the initial ischemic episode, indicating that the late increase in COX-2 activity is involved in the delayed occurrence of oxidative damage in the hippocampus after global ischemia. Interestingly, either selective inhibition of COX-2 with rofecoxib or inhibition of COX-1 with valeryl salicylate significantly increased the number of healthy neurons in the hippocampal CA1 sector even when the treatment began 6 h after ischemia. These results provide the first evidence that both COX isoforms are involved in the progression of neuronal damage following global cerebral ischemia, and have important implications for the potential therapeutic use of COX inhibitors in cerebral ischemia.
Figures



Similar articles
-
Neuroprotective efficacy of nimesulide against hippocampal neuronal damage following transient forebrain ischemia.Eur J Pharmacol. 2002 Oct 25;453(2-3):189-95. doi: 10.1016/s0014-2999(02)02422-6. Eur J Pharmacol. 2002. PMID: 12398903
-
Involvement of cyclooxygenase-derived prostaglandin E2 and nitric oxide in the protection of rat pancreas afforded by low dose of lipopolysaccharide.J Physiol Pharmacol. 2001 Mar;52(1):107-26. J Physiol Pharmacol. 2001. PMID: 11321505
-
The highly selective cyclooxygenase-2 inhibitor DFU is neuroprotective when given several hours after transient cerebral ischemia in gerbils.Brain Res. 2002 Feb 15;927(2):212-5. doi: 10.1016/s0006-8993(01)03358-3. Brain Res. 2002. PMID: 11821016
-
Amelioration of hippocampal neuronal damage after transient forebrain ischemia in cyclooxygenase-2-deficient mice.J Cereb Blood Flow Metab. 2004 Jan;24(1):107-13. doi: 10.1097/01.WCB.0000100065.36077.4A. J Cereb Blood Flow Metab. 2004. PMID: 14688622
-
Cyclooxygenase inhibition in ischemic brain injury.Curr Pharm Des. 2008;14(14):1401-18. doi: 10.2174/138161208784480216. Curr Pharm Des. 2008. PMID: 18537663 Review.
Cited by
-
Molecular basis of etiological implications in Alzheimer's disease: focus on neuroinflammation.Mol Neurobiol. 2013 Dec;48(3):412-28. doi: 10.1007/s12035-013-8428-4. Epub 2013 Feb 19. Mol Neurobiol. 2013. PMID: 23420079 Review.
-
Cyclooxygenases-1 and -2 differentially modulate leukocyte recruitment into the inflamed brain.Pharmacogenomics J. 2010 Oct;10(5):448-57. doi: 10.1038/tpj.2009.68. Epub 2009 Dec 29. Pharmacogenomics J. 2010. PMID: 20038958 Free PMC article.
-
Liver X Receptor as a Possible Drug Target for Blood-Brain Barrier Integrity.Adv Pharm Bull. 2022 May;12(3):466-475. doi: 10.34172/apb.2022.050. Epub 2021 Aug 14. Adv Pharm Bull. 2022. PMID: 35935038 Free PMC article. Review.
-
Prophylactic, prandial rofecoxib treatment lacks efficacy against acute PTZ-induced seizure generation and kindling acquisition.Epilepsia. 2011 Feb;52(2):273-83. doi: 10.1111/j.1528-1167.2010.02889.x. Epub 2011 Jan 10. Epilepsia. 2011. PMID: 21219314 Free PMC article.
-
Neuroinflammation: friend and foe for ischemic stroke.J Neuroinflammation. 2019 Jul 10;16(1):142. doi: 10.1186/s12974-019-1516-2. J Neuroinflammation. 2019. PMID: 31291966 Free PMC article. Review.
References
-
- Akaike A, Kaneko S, Tamura Y, Nakata N, Shiomi H, Ushikubi F, Narumiya S. Prostaglandin E2 protects cultured neurons against N-methyl-D-aspartate receptor-mediated glutamate cytotoxicity. Brain Res. 1994;663:237–243. - PubMed
-
- Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Meth. Enzymol. 1985;113:548–555. - PubMed
-
- Aruoma OI, Halliwell B, Aeschbach R, Löliger J. Antioxidant and prooxidant properties of active rosemary constituents: carnosol and carnosoic acid. Xenobiotica. 1992;22:257–268. - PubMed
-
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. - PubMed
-
- Bhattacharyya DK, Lecomte M, Dunn J, Morgans DJ, Smith WL. Selective inhibition of prostaglandin endoperoxide synthase-1 (cyclooxygenase-1) by valerylsalicylic acid. Arch. Biochem. Biophys. 1995;317:19–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

