Use of mouse models to evaluate the persistence, safety, and immune modulation capacities of lactic acid bacteria
- PMID: 12853407
- PMCID: PMC164262
- DOI: 10.1128/cdli.10.4.696-701.2003
Use of mouse models to evaluate the persistence, safety, and immune modulation capacities of lactic acid bacteria
Abstract
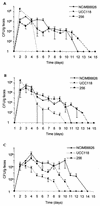
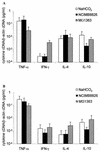
Recent clinical and experimental observations showed that specific probiotic microorganisms may provide therapeutic benefits in inflammatory bowel disease. However, a rigorous screening for new candidate probiotic strains with optimized therapeutic properties necessitates also determining possible adverse interactions with the host, particularly in individuals who are not healthy. We have evaluated the persistence of strains of lactic acid bacteria (LAB) in the digestive tracts of mice, their immunomodulation capacity, and their safety in healthy animals and in a colitis model. Following daily administration of 10(9) CFU of viable LAB orally, intragastrically, or intrarectally, the animals' feces were examined for bacterial excretion and cytokines were quantified in intestinal samples by quantitative reverse transcription-PCR. The level of bacterial translocation was assessed in healthy mice and in mice suffering from colitis induced by 2,4,6-trinitrobenzene sulfonic acid (TNBS). Irrespective of the route of administration, the potential probiotic strain Lactobacillus plantarum NCIMB8826 was found to persist for up to 10 days in the digestive tracts of mice. This strain did not induce detrimental effects in healthy or in TNBS-treated animals, as was reflected by the absence of weight loss, intestinal inflammation, modification of cytokine levels in the ileum and colon (healthy mice), and bacterial dissemination (healthy and colitic animals). Moreover, the translocation of endogenous microflora to the mesenteric lymph nodes and spleen was greatly reduced in the TNBS-treated mice after administration of LAB. This property, together with the strain's persistence capacity and innocuousness renders L. plantarum NCIMB8826 an attractive candidate as a probiotic to be used in the prevention or treatment of chronic inflammation.
Figures
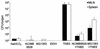
Similar articles
-
Selecting lactic acid bacteria for their safety and functionality by use of a mouse colitis model.Appl Environ Microbiol. 2006 Sep;72(9):5799-805. doi: 10.1128/AEM.00109-06. Appl Environ Microbiol. 2006. PMID: 16957197 Free PMC article.
-
Gene expression profiling identifies mechanisms of protection to recurrent trinitrobenzene sulfonic acid colitis mediated by probiotics.Inflamm Bowel Dis. 2012 Aug;18(8):1424-33. doi: 10.1002/ibd.22849. Epub 2011 Dec 11. Inflamm Bowel Dis. 2012. PMID: 22162025
-
Bacteriocin biosynthesis contributes to the anti-inflammatory capacities of probiotic Lactobacillus plantarum.Benef Microbes. 2018 Feb 27;9(2):333-344. doi: 10.3920/BM2017.0096. Epub 2017 Oct 25. Benef Microbes. 2018. PMID: 29065706
-
[Lactic acid bacteria and health: are probiotics safe for human?].Postepy Hig Med Dosw (Online). 2014 Nov 17;68:1325-34. doi: 10.5604/17322693.1129120. Postepy Hig Med Dosw (Online). 2014. PMID: 25404621 Review. Polish.
-
Probiotics: Isolated bacteria strain or mixtures of different strains? Two different approaches in the use of probiotics as therapeutics.Drugs Today (Barc). 2003 Aug;39(8):565-97. doi: 10.1358/dot.2003.39.8.799406. Drugs Today (Barc). 2003. PMID: 14566382 Review.
Cited by
-
The gut microbiome, resistome, and mycobiome in preterm newborn infants and mouse pups: lack of lasting effects by antimicrobial therapy or probiotic prophylaxis.Gut Pathog. 2024 May 12;16(1):27. doi: 10.1186/s13099-024-00616-w. Gut Pathog. 2024. PMID: 38735967 Free PMC article.
-
Identification of Valerate as Carrying Capacity Modulator by Analyzing Lactiplantibacillus plantarum Colonization of Colonic Microbiota in vitro.Front Microbiol. 2022 May 30;13:910609. doi: 10.3389/fmicb.2022.910609. eCollection 2022. Front Microbiol. 2022. PMID: 35722334 Free PMC article.
-
Utilizing Probiotics for the Prevention and Treatment of Gastrointestinal Diseases.Front Microbiol. 2021 Aug 9;12:689958. doi: 10.3389/fmicb.2021.689958. eCollection 2021. Front Microbiol. 2021. PMID: 34434175 Free PMC article. Review.
-
Challenges in the production and use of probiotics as therapeuticals in cancer treatment or prevention.J Ind Microbiol Biotechnol. 2021 Dec 23;48(9-10):kuab052. doi: 10.1093/jimb/kuab052. J Ind Microbiol Biotechnol. 2021. PMID: 34427674 Free PMC article. Review.
-
Molecular insights into probiotic mechanisms of action employed against intestinal pathogenic bacteria.Gut Microbes. 2020 Nov 9;12(1):1831339. doi: 10.1080/19490976.2020.1831339. Gut Microbes. 2020. PMID: 33112695 Free PMC article. Review.
References
-
- Aguirre, M., and M. D. Collins. 1993. Lactic acid bacteria and human clinical infection. J. Appl. Bacteriol. 75:95-107. - PubMed
-
- Ameho, C. K., A. A. Adjei, E. K. Harrison, K. Takeshita, T. Morioka, Y. Arakaki, E. Ito, I. Suzuki, A. D. Kulkarni, A. Kawajiri, and S. Yamamoto. 1997. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis. Gut 41:487-493. - PMC - PubMed
-
- Bayer, A. S., A. W. Chow, D. Betts, and L. B. Guze. 1978. Lactobacillemia—report of nine cases. Important clinical and therapeutic considerations. Am. J. Med. 64:808-813. - PubMed
-
- Berg, R. D. 1999. Bacterial translocation from the gastrointestinal tract. Adv. Exp. Med. Biol. 473:11-30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

