Hrs regulates early endosome fusion by inhibiting formation of an endosomal SNARE complex
- PMID: 12847087
- PMCID: PMC2172712
- DOI: 10.1083/jcb.200302083
Hrs regulates early endosome fusion by inhibiting formation of an endosomal SNARE complex
Abstract
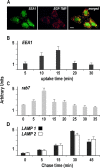
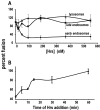
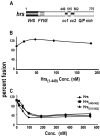
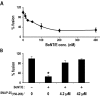
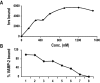
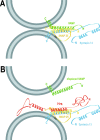
Movement through the endocytic pathway occurs principally via a series of membrane fusion and fission reactions that allow sorting of molecules to be recycled from those to be degraded. Endosome fusion is dependent on SNARE proteins, although the nature of the proteins involved and their regulation has not been fully elucidated. We found that the endosome-associated hepatocyte responsive serum phosphoprotein (Hrs) inhibited the homotypic fusion of early endosomes. A region of Hrs predicted to form a coiled coil required for binding the Q-SNARE, SNAP-25, mimicked the inhibition of endosome fusion produced by full-length Hrs, and was sufficient for endosome binding. SNAP-25, syntaxin 13, and VAMP2 were bound from rat brain membranes to the Hrs coiled-coil domain. Syntaxin 13 inhibited early endosomal fusion and botulinum toxin/E inhibition of early endosomal fusion was reversed by addition of SNAP-25(150-206), confirming a role for syntaxin 13, and establishing a role for SNAP-25 in endosomal fusion. Hrs inhibited formation of the syntaxin 13-SNAP-25-VAMP2 complex by displacing VAMP2 from the complex. These data suggest that SNAP-25 is a receptor for Hrs on early endosomal membranes and that the binding of Hrs to SNAP-25 on endosomal membranes inhibits formation of a SNARE complex required for homotypic endosome fusion.
Figures
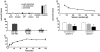





Similar articles
-
Ca2+ and N-ethylmaleimide-sensitive factor differentially regulate disassembly of SNARE complexes on early endosomes.J Biol Chem. 2004 Apr 30;279(18):18270-6. doi: 10.1074/jbc.M400093200. Epub 2004 Feb 9. J Biol Chem. 2004. PMID: 14769786
-
Syntaxin 7 and VAMP-7 are soluble N-ethylmaleimide-sensitive factor attachment protein receptors required for late endosome-lysosome and homotypic lysosome fusion in alveolar macrophages.Mol Biol Cell. 2000 Jul;11(7):2327-33. doi: 10.1091/mbc.11.7.2327. Mol Biol Cell. 2000. PMID: 10888671 Free PMC article.
-
A SNARE complex mediating fusion of late endosomes defines conserved properties of SNARE structure and function.EMBO J. 2000 Dec 1;19(23):6453-64. doi: 10.1093/emboj/19.23.6453. EMBO J. 2000. PMID: 11101518 Free PMC article.
-
Biogenesis of the sorting endosome: the role of Rab5.Traffic. 2000 Sep;1(9):695-701. doi: 10.1034/j.1600-0854.2000.010902.x. Traffic. 2000. PMID: 11208157 Review.
-
SNARE protein structure and function.Annu Rev Cell Dev Biol. 2003;19:493-517. doi: 10.1146/annurev.cellbio.19.110701.155609. Annu Rev Cell Dev Biol. 2003. PMID: 14570579 Review.
Cited by
-
Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes.Neuron. 2006 Dec 7;52(5):817-30. doi: 10.1016/j.neuron.2006.09.040. Neuron. 2006. PMID: 17145503 Free PMC article.
-
Comparative analysis of ESCRT-I, ESCRT-II and ESCRT-III function in Drosophila by efficient isolation of ESCRT mutants.J Cell Sci. 2009 Jul 15;122(Pt 14):2413-23. doi: 10.1242/jcs.046391. J Cell Sci. 2009. PMID: 19571114 Free PMC article.
-
UBE4B levels are correlated with clinical outcomes in neuroblastoma patients and with altered neuroblastoma cell proliferation and sensitivity to epidermal growth factor receptor inhibitors.Cancer. 2013 Feb 15;119(4):915-23. doi: 10.1002/cncr.27785. Epub 2012 Sep 18. Cancer. 2013. PMID: 22990745 Free PMC article.
-
i-SNAREs: inhibitory SNAREs that fine-tune the specificity of membrane fusion.J Cell Biol. 2004 Jan 5;164(1):79-88. doi: 10.1083/jcb.200307066. Epub 2003 Dec 29. J Cell Biol. 2004. PMID: 14699088 Free PMC article.
-
UBE4B protein couples ubiquitination and sorting machineries to enable epidermal growth factor receptor (EGFR) degradation.J Biol Chem. 2014 Jan 31;289(5):3026-39. doi: 10.1074/jbc.M113.495671. Epub 2013 Dec 16. J Biol Chem. 2014. PMID: 24344129 Free PMC article.
References
-
- Advani, R.J., H.R. Bae, J.B. Bock, D.S. Chao, Y.C. Doung, R. Prekeris, J.S. Yoo, and R.H. Scheller. 1998. Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J. Biol. Chem. 273:10317–10324. - PubMed
-
- Asao, H., Y. Sasaki, T. Arita, N. Tanaka, K. Endo, H. Kasai, T. Takeshita, Y. Endo, T. Fujita, and K. Sugamura. 1997. Hrs is associated with STAM, a signal-transducing adaptor molecule. Its suppressive effect on cytokine-induced cell growth. J. Biol. Chem. 272:32785–32791. - PubMed
-
- Banerjee, A., J.A. Kowalchyk, B.R. DasGupta, and T.F. Martin. 1996. SNAP-25 is required for a late postdocking step in Ca2+-dependent exocytosis. J. Biol. Chem. 271:20227–20230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

