Four-dimensional migratory coordinates of GABAergic interneurons in the developing mouse cortex
- PMID: 12843285
- PMCID: PMC6741259
- DOI: 10.1523/JNEUROSCI.23-13-05805.2003
Four-dimensional migratory coordinates of GABAergic interneurons in the developing mouse cortex
Abstract
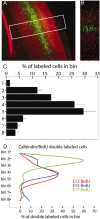
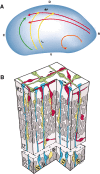
We have used time-lapse multiphoton microscopy to map the migration and settling pattern of GABAergic interneurons that originate in the ganglionic eminence of the ventral forebrain and incorporate into the neocortex of the cerebral hemispheres. Imaging of the surface of the cerebral hemispheres in both explant cultures and brains of living mouse embryos revealed that GABAergic interneurons migrating within the marginal zone originate from three different sources and migrate via distinct and independent streams. After reaching their areal destination, interneurons descend into the underlying cortex to assume positions with isochronically generated, radially derived neurons. The dynamics and pattern of cell migration in the marginal zone (see movies, available at www.jneurosci.org) suggest that the three populations of interneurons respond selectively to distinct local cues for directing their migration to the appropriate areas and layers of the neocortex. This approach opens a new avenue for study of normal and abnormal neuronal migration in their native environment and indicate that interneurons have specific programs for their areal and laminar deployment.
Figures



Similar articles
-
Multidirectional and multizonal tangential migration of GABAergic interneurons in the developing cerebral cortex.Development. 2006 Jun;133(11):2167-76. doi: 10.1242/dev.02382. Epub 2006 May 3. Development. 2006. PMID: 16672340
-
Cell migration from the ganglionic eminences is required for the development of hippocampal GABAergic interneurons.Neuron. 2000 Dec;28(3):727-40. doi: 10.1016/s0896-6273(00)00149-5. Neuron. 2000. PMID: 11163262
-
Cajal-Retzius cells in the mouse: transcription factors, neurotransmitters, and birthdays suggest a pallial origin.Brain Res Dev Brain Res. 2003 Mar 14;141(1-2):39-53. doi: 10.1016/s0165-3806(02)00641-7. Brain Res Dev Brain Res. 2003. PMID: 12644247
-
Intracortical multidirectional migration of cortical interneurons.Novartis Found Symp. 2007;288:116-25; discussion 125-9, 276-81. Novartis Found Symp. 2007. PMID: 18494255 Review.
-
Neuronal migration in the developing cerebral cortex: observations based on real-time imaging.Cereb Cortex. 2003 Jun;13(6):607-11. doi: 10.1093/cercor/13.6.607. Cereb Cortex. 2003. PMID: 12764035 Review.
Cited by
-
Altered migratory behavior of interneurons in a model of cortical dysplasia: the influence of elevated GABAA activity.Cereb Cortex. 2014 Sep;24(9):2297-308. doi: 10.1093/cercor/bht073. Epub 2013 Apr 9. Cereb Cortex. 2014. PMID: 23574639 Free PMC article.
-
JNK Signaling Regulates Cellular Mechanics of Cortical Interneuron Migration.eNeuro. 2020 Aug 20;7(4):ENEURO.0132-20.2020. doi: 10.1523/ENEURO.0132-20.2020. Print 2020 Jul/Aug. eNeuro. 2020. PMID: 32737185 Free PMC article.
-
Lineage Relationships Do Not Drive MGE/PoA-Derived Interneuron Clustering in the Brain.Neuron. 2016 Oct 5;92(1):52-58. doi: 10.1016/j.neuron.2016.09.034. Neuron. 2016. PMID: 27710790 Free PMC article.
-
Connexin 43 controls the multipolar phase of neuronal migration to the cerebral cortex.Proc Natl Acad Sci U S A. 2012 May 22;109(21):8280-5. doi: 10.1073/pnas.1205880109. Epub 2012 May 7. Proc Natl Acad Sci U S A. 2012. PMID: 22566616 Free PMC article.
-
Tangential networks of precocious neurons and early axonal outgrowth in the embryonic human forebrain.J Neurosci. 2005 Mar 16;25(11):2781-92. doi: 10.1523/JNEUROSCI.4770-04.2005. J Neurosci. 2005. PMID: 15772338 Free PMC article.
References
-
- Alcantara S, Ferrer I, Soriano E ( 1993) Postnatal development of parvalbumin and calbindin D28K immunoreactivities in the cerebral cortex of the rat. Anat Embryol (Berl) 188: 63-73. - PubMed
-
- Alcantara S, de Lecea L, Del Rio JA, Ferrer I, Soriano E ( 1996) Transient colocalization of parvalbumin and calbindin D28k in the postnatal cerebral cortex: evidence for a phenotypic shift in developing nonpyramidal neurons. Eur J Neurosci 8: 1329-1339. - PubMed
-
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL ( 1997) Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science 278: 474-476. - PubMed
-
- Anderson SA, Marin O, Horn C, Jennings K, Rubenstein JL ( 2001) Distinct cortical migrations from the medial and lateral ganglionic eminences. Development 128: 353-363. - PubMed
-
- Angevine Jr JB, Sidman RL ( 1961) Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature 192: 766-768. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

