Iris pigment epithelium expressing CD86 (B7-2) directly suppresses T cell activation in vitro via binding to cytotoxic T lymphocyte-associated antigen 4
- PMID: 12835481
- PMCID: PMC2196085
- DOI: 10.1084/jem.20030097
Iris pigment epithelium expressing CD86 (B7-2) directly suppresses T cell activation in vitro via binding to cytotoxic T lymphocyte-associated antigen 4
Abstract
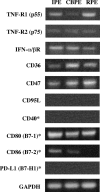
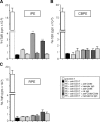
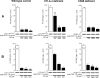
A monolayer of pigment epithelium (PE) lines the iris PE (IPE), ciliary body PE, and retina PE of the inner eye, an immune-privileged site. These neural crest-derived epithelial cells participate in ocular immune privilege through poorly defined molecular mechanisms. Murine PE cells cultured from different ocular tissues suppress T cell activation by differing mechanisms. In particular, IPE cells suppress primarily via direct cell to cell contact. By examining surface expression of numerous candidate molecules (tumor necrosis factor receptor [TNFR]1, TNFR2, CD36, CD40, CD47, CD80, CD86, PD-L1, CD95 ligand, and type I interferon receptor), we report that IPE cells uniquely express on their surface the costimulatory molecule CD86. When IPE were blocked with anti-CD86 or were derived from CD80/CD86 (but not CD80) knockout (KO) mice, the cells displayed reduced capacity to suppress T cell activation. IPE also failed to suppress activation of T cells in the presence of cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) immunoglobulin or if the T cells were obtained from CTLA-4 (but not CD28) KO mice. We conclude that iris pigment epithelial cells constitutively express cell surface CD86, which enables the cells to contact inhibit T cells via direct interaction with CTLA-4. Thus, ocular immune privilege is achieved in part by subversion of molecules that are usually used for conventional immune costimulation.
Figures
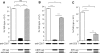


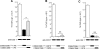
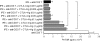
Similar articles
-
CTLA-4+CD8+ T cells that encounter B7-2+ iris pigment epithelial cells express their own B7-2 to achieve global suppression of T cell activation.J Immunol. 2004 Apr 1;172(7):4184-94. doi: 10.4049/jimmunol.172.7.4184. J Immunol. 2004. PMID: 15034031
-
B7+ iris pigment epithelial cells convert T cells into CTLA-4+, B7-expressing CD8+ regulatory T cells.Invest Ophthalmol Vis Sci. 2006 Dec;47(12):5376-84. doi: 10.1167/iovs.05-1354. Invest Ophthalmol Vis Sci. 2006. PMID: 17122127
-
Participation of pigment epithelium in ocular immune privilege. 3. Epithelia cultured from iris, ciliary body, and retina suppress T-cell activation by partially non-overlapping mechanisms.Ocul Immunol Inflamm. 2003 Jun;11(2):91-105. doi: 10.1076/ocii.11.2.91.15914. Ocul Immunol Inflamm. 2003. PMID: 14533028
-
[Costimulatory molecules in autoimmunity: role of CD28/CTLA4-CD80/CD86].Nihon Rinsho. 1997 Jun;55(6):1419-24. Nihon Rinsho. 1997. PMID: 9200926 Review. Japanese.
-
The B7-CD28 superfamily.Nat Rev Immunol. 2002 Feb;2(2):116-26. doi: 10.1038/nri727. Nat Rev Immunol. 2002. PMID: 11910893 Review.
Cited by
-
Broad spectrum antiangiogenic treatment for ocular neovascular diseases.PLoS One. 2010 Sep 1;5(9):e12515. doi: 10.1371/journal.pone.0012515. PLoS One. 2010. PMID: 20824139 Free PMC article.
-
Suppression of interleukin-17-producing T-helper 17 cells by retinal pigment epithelial cells.Jpn J Ophthalmol. 2011 Sep;55(5):565-575. doi: 10.1007/s10384-011-0064-9. Epub 2011 Jul 13. Jpn J Ophthalmol. 2011. PMID: 21750969
-
Mechanisms of immune privilege in the anterior segment of the eye: what we learn from corneal transplantation.J Ocul Biol Dis Infor. 2008 Dec;1(2-4):94-100. doi: 10.1007/s12177-008-9010-6. Epub 2008 Aug 8. J Ocul Biol Dis Infor. 2008. PMID: 20072639 Free PMC article.
-
The Cellular Composition of the Uveal Immune Environment.Front Med (Lausanne). 2021 Oct 29;8:721953. doi: 10.3389/fmed.2021.721953. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34778287 Free PMC article. Review.
-
Re-programming immunosurveillance in persistent non-infectious ocular inflammation.Prog Retin Eye Res. 2018 Jul;65:93-106. doi: 10.1016/j.preteyeres.2018.03.001. Epub 2018 Mar 9. Prog Retin Eye Res. 2018. PMID: 29530739 Free PMC article. Review.
References
-
- Streilein, J.W. 1993. Immune privilege as the result of local tissue barriers and immunosuppressive microenvironments. Curr. Opin. Immunol. 5:428–432. - PubMed
-
- Streilein, J.W. 1996. Ocular immune privilege and the faustian dilemma. Invest. Ophthalmol. Vis. Sci. 37:1940–1950. - PubMed
-
- Streilein, J.W., B.R. Ksander, and A.W. Taylor. 1997. Immune deviation in relation to ocular immune privilege. J. Immunol. 158:3557–3560. - PubMed
-
- Taylor, A.W. 1999. Ocular immunosuppressive microenvironment. Chem. Immunol. 73:72–89. - PubMed
-
- Streilein, J.W. 1997. Molecular basis of ACAID. Ocul. Immunol. Inflamm. 5:217–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

