Location of structural transitions in an isotopically labeled lung surfactant SP-B peptide by IRRAS
- PMID: 12829488
- PMCID: PMC1303089
- DOI: 10.1016/S0006-3495(03)74478-6
Location of structural transitions in an isotopically labeled lung surfactant SP-B peptide by IRRAS
Abstract
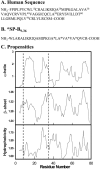
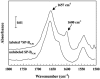
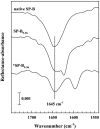
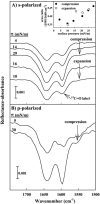
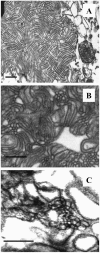
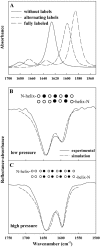
Pulmonary surfactant, a lipid/protein complex that lines the air/water interface in the mammalian lung, functions to reduce the work of breathing. Surfactant protein B (SP-B) is a small, hydrophobic protein that is an essential component of this mixture. Structure-function relationships of SP-B are currently under investigation as the protein and its peptide analogs are being incorporated into surfactant replacement therapies. Knowledge of the structure of SP-B and its related peptides in bulk and monolayer phases will facilitate the design of later generation therapeutic agents. Prior infrared reflection-absorption spectroscopic studies reported notable, reversible surface pressure-induced antiparallel beta-sheet formation in a synthetic peptide derived from human SP-B, residues 9-36 (SP-B(9-36)). In the current work, infrared reflection-absorption spectroscopy is applied in conjunction with isotopic labeling to detect the site and pressure dependence of the conformational change. SP-B(9-36), synthesized with (13)C=O-labeled Ala residues in positions 26, 28, 30, and 32, shifted the beta-sheet marker band to approximately 1600 cm(-1) and thus immediately identified this structural element within the labeled region. Surface pressure-induced alterations in the relative intensities of Amide I band constituents are interpreted using a semiempirical transition dipole coupling model. In addition, electron micrographs reveal the formation of tubular myelin structures from in vitro preparations using SP-B(9-36) in place of porcine SP-B indicating that the peptide has the potential to mimic this property of the native protein.
Figures
Similar articles
-
Secondary structure and lipid interactions of the N-terminal segment of pulmonary surfactant SP-C in Langmuir films: IR reflection-absorption spectroscopy and surface pressure studies.Biochemistry. 2002 Jul 2;41(26):8385-95. doi: 10.1021/bi020129g. Biochemistry. 2002. PMID: 12081487
-
Secondary structure in lung surfactant SP-B peptides: IR and CD studies of bulk and monolayer phases.Biochim Biophys Acta. 2001 Mar 9;1511(1):99-112. doi: 10.1016/s0005-2736(00)00387-4. Biochim Biophys Acta. 2001. PMID: 11248209
-
Effect of hydrophobic surfactant proteins SP-B and SP-C on binary phospholipid monolayers: II. Infrared external reflectance-absorption spectroscopy.Biophys J. 2003 Jan;84(1):326-40. doi: 10.1016/S0006-3495(03)74853-X. Biophys J. 2003. PMID: 12524286 Free PMC article.
-
Hydrophobic surfactant proteins and their analogues.Neonatology. 2007;91(4):303-10. doi: 10.1159/000101346. Epub 2007 Jun 7. Neonatology. 2007. PMID: 17575474 Review.
-
Protein-lipid interactions and surface activity in the pulmonary surfactant system.Chem Phys Lipids. 2006 Jun;141(1-2):105-18. doi: 10.1016/j.chemphyslip.2006.02.017. Epub 2006 Mar 20. Chem Phys Lipids. 2006. PMID: 16600200 Review.
Cited by
-
Infrared reflection-absorption spectroscopy: principles and applications to lipid-protein interaction in Langmuir films.Biochim Biophys Acta. 2010 Apr;1798(4):788-800. doi: 10.1016/j.bbamem.2009.11.024. Epub 2010 Jan 4. Biochim Biophys Acta. 2010. PMID: 20004639 Free PMC article. Review.
-
Critical structure-function determinants within the N-terminal region of pulmonary surfactant protein SP-B.Biophys J. 2006 Jan 1;90(1):238-49. doi: 10.1529/biophysj.105.073403. Epub 2005 Oct 7. Biophys J. 2006. PMID: 16214863 Free PMC article.
-
Biophysical approaches for exploring lipopeptide-lipid interactions.Biochimie. 2020 Mar;170:173-202. doi: 10.1016/j.biochi.2020.01.009. Epub 2020 Jan 21. Biochimie. 2020. PMID: 31978418 Free PMC article. Review.
-
Structure-function correlations of pulmonary surfactant protein SP-B and the saposin-like family of proteins.Eur Biophys J. 2013 Mar;42(2-3):209-22. doi: 10.1007/s00249-012-0858-9. Epub 2012 Sep 21. Eur Biophys J. 2013. PMID: 22996193 Review.
-
Emulation of the structure of the Saposin protein fold by a lung surfactant peptide construct of surfactant Protein B.PLoS One. 2022 Nov 3;17(11):e0276787. doi: 10.1371/journal.pone.0276787. eCollection 2022. PLoS One. 2022. PMID: 36327300 Free PMC article.
References
-
- Andersson, M., T. Curstedt, H. Jornvall, and J. Johansson. 1995. An amphipathic helical motif common to tumourolytic polypeptide NK-lysin and pulmonary surfactant polypeptide SP-B. FEBS Lett. 362:328–332. - PubMed
-
- Bartlett, G. R. 1959. Phosphorous assay in column chromatography. J. Biol. Chem. 234:466–468. - PubMed
-
- Bi, X., C. R. Flach, J. Perez-Gil, I. Plasencia, D. Andreu, E. Oliveira, and R. Mendelsohn. 2002. Secondary structure and lipid interactions of the N-terminal segment of pulmonary surfactant SP-C in Langmuir films: IR reflection-absorption spectroscopy and surface pressure studies. Biochemistry. 41:8385–8395. - PubMed
-
- Brauner, J. W., C. Dugan, and R. Mendelsohn. 2000. 13C isotope labeling of hydrophobic peptides. Origin of the anomalous intensity distribution in the infrared Amide I spectral region of β-sheet structures. J. Am. Chem. Soc. 122:677–683.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

