Asymmetric localization of flotillins/reggies in preassembled platforms confers inherent polarity to hematopoietic cells
- PMID: 12826615
- PMCID: PMC166213
- DOI: 10.1073/pnas.1331629100
Asymmetric localization of flotillins/reggies in preassembled platforms confers inherent polarity to hematopoietic cells
Abstract
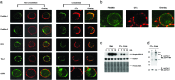
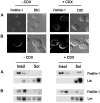
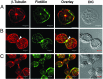
Hematopoietic cells have long been defined as round, nonpolar cells that show uniform distribution of cell surface-associated molecules. However, recent analyses of the immunological synapse and the importance of lipid microdomains in signaling have shed new light on the aspect of lymphocyte polarization during the activation processes, but none of the molecules implicated so far in either the activation process or the microdomain residency are known to have a preferential localization in nonactivated cells. Chemical crosslinking and fluorescence resonance energy transfer methods have allowed the visualization of certain glycosylphosphatidylinositol-anchored proteins in lipid rafts but so far no microdomain resident protein has been shown to exist as visible stable platforms in the membrane. We report here that two lipid microdomain resident proteins, flotillins/reggies, form preassembled platforms in hematopoietic cells. These platforms recruit signaling molecules upon activation through lipid rafts. The preassembled platforms significantly differ from the canonical cholesterol-dependent "lipid rafts," as they are resistant to cholesterol-disrupting agents. Most evidence for the functional relevance of microdomains in living cells remains indirect. Using laser scanning confocal microscopy, we show that these proteins exist as stable, microscopically patent domains localizing asymmetrically to one pole of the cell. We present evidence that the asymmetric concentration of these microdomain resident proteins is built up during cytokinesis.
Figures
Similar articles
-
Membrane cholesterol regulates LFA-1 function and lipid raft heterogeneity.Blood. 2003 Jul 1;102(1):215-22. doi: 10.1182/blood-2002-10-3195. Epub 2003 Mar 13. Blood. 2003. PMID: 12637320
-
Lipid microdomain clustering induces a redistribution of antigen recognition and adhesion molecules on human T lymphocytes.J Immunol. 2002 Mar 15;168(6):2737-44. doi: 10.4049/jimmunol.168.6.2737. J Immunol. 2002. PMID: 11884440
-
Dynamic association of CD45 with detergent-insoluble microdomains in T lymphocytes.J Immunol. 2002 Nov 1;169(9):5036-42. doi: 10.4049/jimmunol.169.9.5036. J Immunol. 2002. PMID: 12391219
-
Update on lipid membrane microdomains.Curr Opin Clin Nutr Metab Care. 2008 Mar;11(2):106-12. doi: 10.1097/MCO.0b013e3282f44c2c. Curr Opin Clin Nutr Metab Care. 2008. PMID: 18301084 Review.
-
Lipid rafts and regulation of the cytoskeleton during T cell activation.Philos Trans R Soc Lond B Biol Sci. 2005 Sep 29;360(1461):1663-72. doi: 10.1098/rstb.2005.1704. Philos Trans R Soc Lond B Biol Sci. 2005. PMID: 16147530 Free PMC article. Review.
Cited by
-
Membrane-microdomain localization of amyloid β-precursor protein (APP) C-terminal fragments is regulated by phosphorylation of the cytoplasmic Thr668 residue.J Biol Chem. 2012 Jun 1;287(23):19715-24. doi: 10.1074/jbc.M111.334847. Epub 2012 Apr 17. J Biol Chem. 2012. PMID: 22511769 Free PMC article.
-
Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms.Science. 2004 Jul 2;305(5680):61-6. doi: 10.1126/science.1097931. Epub 2004 May 27. Science. 2004. PMID: 15166316 Free PMC article.
-
Distinct lipid rafts in subdomains from human placental apical syncytiotrophoblast membranes.J Membr Biol. 2008 Jul-Aug;224(1-3):21-31. doi: 10.1007/s00232-008-9125-5. Epub 2008 Sep 20. J Membr Biol. 2008. PMID: 18807084
-
Mechanisms for the formation of membranous nanostructures in cell-to-cell communication.Cell Mol Biol Lett. 2009;14(4):636-56. doi: 10.2478/s11658-009-0018-0. Epub 2009 Jun 25. Cell Mol Biol Lett. 2009. PMID: 19554268 Free PMC article. Review.
-
Flotillins promote T cell receptor sorting through a fast Rab5-Rab11 endocytic recycling axis.Nat Commun. 2019 Sep 26;10(1):4392. doi: 10.1038/s41467-019-12352-w. Nat Commun. 2019. PMID: 31558725 Free PMC article.
References
-
- Anton van der Merwe, P., Davis, S. J., Shaw, A. S. & Dustin, M. L. (2000) Semin. Immunol. 12 5-21. - PubMed
-
- Bromley, S. K., Burack, W. R., Johnson, K. G., Somersalo, K., Sims, T. N., Sumen, C., Davis, M. M., Shaw, A. S., Allen, P. M. & Dustin, M. L. (2001) Annu. Rev. Immunol. 19 375-396. - PubMed
-
- Simons, K. & Toomre, D. (2000) Nat. Rev. Mol. Cell Biol. 1 31-39. - PubMed
-
- Dustin, M. L. & Chan, A. C. (2000) Cell 103 283-294. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

