Interleukin-2-independent proliferation of human melanoma-reactive T lymphocytes transduced with an exogenous IL-2 gene is stimulation dependent
- PMID: 12806273
- PMCID: PMC2553215
- DOI: 10.1097/00002371-200305000-00003
Interleukin-2-independent proliferation of human melanoma-reactive T lymphocytes transduced with an exogenous IL-2 gene is stimulation dependent
Abstract
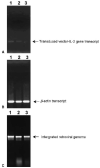
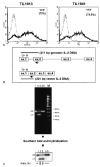
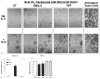
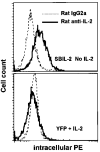
A major obstacle limiting the efficacy of adoptive T-cell transfer (adoptive immunotherapy) to treat patients with cancer is the short survival of the transferred cells. These in vitro activated T cells depend on the growth factor, interleukin (IL)-2, and may undergo apoptosis in vivo when they are transferred. The authors previously reported that the need for an exogenous source of IL-2 could be abrogated in vitro by retrovirally transducing antitumor T lymphocytes with an exogenous IL-2 gene. Here they report that this growth of IL-2 transductants depended on restimulation of the T-cell receptor complex and appeared to be regulated at the transcriptional level of the transduced IL-2 gene. The transduced IL-2 transcript was barely detectable in IL-2-transductants just before they died without restimulation, and they expressed a low level of the CD25 molecule, the alpha chain of the IL-2 trimeric receptor complex. Melanoma-specific tumor-infiltrating lymphocytes (either bulk or CD8+ cells alone), when transduced with an IL-2 retroviral vector, could produce IL-2 upon tumor stimulation and proliferated after the destruction of autologous tumor cells in the absence of added IL-2. Control vector-transduced tumor-infiltrating lymphocytes failed to do so under the same conditions. These findings provide a foundation for the development of clinical efforts to adoptively transfer melanoma-specific tumor-infiltrating lymphocytes transduced with an IL-2 retroviral vector for the treatment of patients with metastatic melanoma to evaluate the fate and therapeutic effect of these IL-2 gene-modified antitumor T lymphocytes in vivo.
Figures


Similar articles
-
Transduction of an IL-2 gene into human melanoma-reactive lymphocytes results in their continued growth in the absence of exogenous IL-2 and maintenance of specific antitumor activity.J Immunol. 2001 Dec 1;167(11):6356-65. doi: 10.4049/jimmunol.167.11.6356. J Immunol. 2001. PMID: 11714800 Free PMC article.
-
Interleukin-2 gene-transduced human melanoma cells efficiently stimulate MHC-unrestricted and MHC-restricted autologous lymphocytes.Hum Gene Ther. 1994 Sep;5(9):1139-50. doi: 10.1089/hum.1994.5.9-1139. Hum Gene Ther. 1994. PMID: 7833372
-
Prevention of interleukin-2 withdrawal-induced apoptosis in lymphocytes retrovirally cotransduced with genes encoding an antitumor T-cell receptor and an antiapoptotic protein.J Immunother. 2010 Sep;33(7):672-83. doi: 10.1097/CJI.0b013e3181e475cd. J Immunother. 2010. PMID: 20664359 Free PMC article.
-
Potential use of T cell receptor genes to modify hematopoietic stem cells for the gene therapy of cancer.Pathol Oncol Res. 1999;5(1):3-15. doi: 10.1053/paor.1999.0003. Pathol Oncol Res. 1999. PMID: 10079371 Review.
-
Prospects for gene therapy and lymphokine therapy for metastatic melanoma.Ann Plast Surg. 1992 Jan;28(1):114-8. doi: 10.1097/00000637-199201000-00029. Ann Plast Surg. 1992. PMID: 1642399 Review.
Cited by
-
Primary human T lymphocytes engineered with a codon-optimized IL-15 gene resist cytokine withdrawal-induced apoptosis and persist long-term in the absence of exogenous cytokine.J Immunol. 2005 Dec 1;175(11):7226-34. doi: 10.4049/jimmunol.175.11.7226. J Immunol. 2005. PMID: 16301627 Free PMC article.
-
T-cell receptor gene therapy targeting melanoma-associated antigen-A4 inhibits human tumor growth in non-obese diabetic/SCID/γcnull mice.Cancer Sci. 2012 Jan;103(1):17-25. doi: 10.1111/j.1349-7006.2011.02111.x. Epub 2011 Nov 8. Cancer Sci. 2012. PMID: 21951605 Free PMC article.
-
High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation.Mol Ther. 2006 Jan;13(1):151-9. doi: 10.1016/j.ymthe.2005.07.688. Epub 2005 Sep 2. Mol Ther. 2006. PMID: 16140584 Free PMC article.
-
A B-cell lymphoma vaccine using a depot formulation of interleukin-2 induces potent antitumor immunity despite increased numbers of intratumoral regulatory T cells.Cancer Immunol Immunother. 2010 Apr;59(4):519-27. doi: 10.1007/s00262-009-0768-6. Epub 2009 Sep 19. Cancer Immunol Immunother. 2010. PMID: 19768458 Free PMC article.
-
Cancer regression in patients with metastatic melanoma after the transfer of autologous antitumor lymphocytes.Proc Natl Acad Sci U S A. 2004 Oct 5;101 Suppl 2(Suppl 2):14639-45. doi: 10.1073/pnas.0405730101. Epub 2004 Sep 20. Proc Natl Acad Sci U S A. 2004. PMID: 15381769 Free PMC article. Clinical Trial.
References
-
- Sprent J. T-cell survival and the role of cytokines. Immunol Cell Biol. 2001;79:199–206. - PubMed
-
- Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240:1169–1176. - PubMed
-
- Miyazaki TZ, Liu J, Kawahara A, et al. Three distinct IL-2 signaling pathways mediated by bcl-2, c-myc, and lck cooperate in hematopoietic cell proliferation. Cell. 1995;81:223–231. - PubMed
-
- Lord JD, McIntosh BC, Greenberg PD, et al. The IL-2 receptor promotes proliferation, bcl-2 and bcl-x induction, but not cell viability through the adapter molecule Shc. J Immunol. 1998;161:4627–4633. - PubMed
-
- Lord JD, McIntosh BC, Greenberg PD, et al. The IL-2 receptor promotes lymphocyte proliferation and induction of the c-myc, bcl-2, and bcl-x genes through the trans-activation domain of Stat5. J Immunol. 2000;164:2533–2541. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

