Viability of poliovirus/rhinovirus VPg chimeric viruses and identification of an amino acid residue in the VPg gene critical for viral RNA replication
- PMID: 12805442
- PMCID: PMC164788
- DOI: 10.1128/jvi.77.13.7434-7443.2003
Viability of poliovirus/rhinovirus VPg chimeric viruses and identification of an amino acid residue in the VPg gene critical for viral RNA replication
Abstract
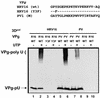
Picornaviral RNA replication utilizes a small virus-encoded protein, termed 3B or VPg, as a primer to initiate RNA synthesis. This priming step requires uridylylation of the VPg peptide by the viral polymerase protein 3D(pol), in conjunction with other viral or host cofactors. In this study, we compared the viral specificity in 3D(pol)-catalyzed uridylylation reactions between poliovirus (PV) and human rhinovirus 16 (HRV16). It was found that HRV16 3D(pol) was able to uridylylate PV VPg as efficiently as its own VPg, but PV 3D(pol) could not uridylylate HRV16 VPg. Two chimeric viruses, PV containing HRV16 VPg (PV/R16-VPg) and HRV16 containing PV VPg (R16/PV-VPg), were constructed and tested for replication capability in H1-HeLa cells. Interestingly, only PV/R16-VPg chimeric RNA produced infectious virus particles upon transfection. No viral RNA replication or cytopathic effect was observed in cells transfected with R16/PV-VPg chimeric RNA, despite the ability of HRV16 3D(pol) to uridylylate PV VPg in vitro. Sequencing analysis of virion RNA isolated from the virus particles generated by PV/R16-VPg chimeric RNA identified a single residue mutation in the VPg peptide (Glu(6) to Val). Reverse genetics confirmed that this mutation was highly compensatory in enhancing replication of the chimeric viral RNA. PV/R16-VPg RNA carrying this mutation replicated with similar kinetics and magnitude to wild-type PV RNA. This cell culture-induced mutation in HRV16 VPg moderately increased its uridylylation by PV 3D(pol) in vitro, suggesting that it might be involved in other function(s) in addition to the direct uridylylation reaction. This study demonstrated the use of chimeric viruses to characterize viral specificity and compatibility in vivo between PV and HRV16 and to identify critical amino acid residue(s) for viral RNA replication.
Figures






Similar articles
-
Biochemical and genetic studies of the VPg uridylylation reaction catalyzed by the RNA polymerase of poliovirus.J Virol. 2003 Jan;77(2):891-904. doi: 10.1128/jvi.77.2.891-904.2003. J Virol. 2003. PMID: 12502805 Free PMC article.
-
Genetic and biochemical studies of poliovirus cis-acting replication element cre in relation to VPg uridylylation.J Virol. 2000 Nov;74(22):10371-80. doi: 10.1128/jvi.74.22.10371-10380.2000. J Virol. 2000. PMID: 11044081 Free PMC article.
-
Human rhinovirus type 14 gain-of-function mutants for oriI utilization define residues of 3C(D) and 3Dpol that contribute to assembly and stability of the picornavirus VPg uridylylation complex.J Virol. 2007 Nov;81(22):12485-95. doi: 10.1128/JVI.00972-07. Epub 2007 Sep 12. J Virol. 2007. PMID: 17855535 Free PMC article.
-
Uridylylation of the genome-linked protein of poliovirus in vitro is dependent upon an endogenous RNA template.Virus Res. 1987 Sep;8(3):193-204. doi: 10.1016/0168-1702(87)90015-3. Virus Res. 1987. PMID: 2825442 Review.
-
Formation and working mechanism of the picornavirus VPg uridylylation complex.Curr Opin Virol. 2014 Dec;9:24-30. doi: 10.1016/j.coviro.2014.09.003. Epub 2014 Sep 19. Curr Opin Virol. 2014. PMID: 25240314 Review.
Cited by
-
Engineered picornavirus VPg-RNA substrates: analysis of a tyrosyl-RNA phosphodiesterase activity.PLoS One. 2011 Mar 7;6(3):e16559. doi: 10.1371/journal.pone.0016559. PLoS One. 2011. PMID: 21408223 Free PMC article.
-
A highly divergent picornavirus in a marine mammal.J Virol. 2008 Jan;82(1):311-20. doi: 10.1128/JVI.01240-07. Epub 2007 Oct 17. J Virol. 2008. PMID: 17942560 Free PMC article.
-
Crystal structure of complete rhinovirus RNA polymerase suggests front loading of protein primer.J Virol. 2005 Jan;79(1):277-88. doi: 10.1128/JVI.79.1.277-288.2005. J Virol. 2005. PMID: 15596823 Free PMC article.
-
Multiple classes of antiviral agents exhibit in vitro activity against human rhinovirus type C.Antimicrob Agents Chemother. 2014;58(3):1546-55. doi: 10.1128/AAC.01746-13. Epub 2013 Dec 23. Antimicrob Agents Chemother. 2014. PMID: 24366736 Free PMC article.
-
Direct interaction between two viral proteins, the nonstructural protein 2C and the capsid protein VP3, is required for enterovirus morphogenesis.PLoS Pathog. 2010 Aug 26;6(8):e1001066. doi: 10.1371/journal.ppat.1001066. PLoS Pathog. 2010. PMID: 20865167 Free PMC article.
References
-
- Arnold, E., J. W. Erickson, G. Shay Fout, E. A. Frankenberger, H.-J. Hecht, M. Luo, M. G. Rossmann, and R. R. Rueckert. 1984. Virion orientation in cubic crystals of human common cold virus HRV14. J. Mol. Biol. 177:417-430. - PubMed
-
- Arnold, J. J., and C. E. Cameron. 2000. Poliovirus RNA-dependent RNA polymerase (3Dpol). Assembly of stable elongation-competent complexes by using a symmetrical primer-template substrate (sym/sub). J. Biol. Chem. 275:5329-5336. - PubMed
-
- Baker, R. T., S. A. Smith, R. Marano, J. McKee, and P. G. Board. 1994. Protein expression using cotranslational fusion and cleavage of ubiquitin. J. Biol. Chem. 269:25381-25386. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

