Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion
- PMID: 12796479
- PMCID: PMC2172955
- DOI: 10.1083/jcb.200211118
Reactive oxygen species as essential mediators of cell adhesion: the oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion
Abstract
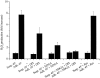
Signal transduction by reactive oxygen species (ROS; "redox signaling") has recently come into focus in cellular biology studies. The signaling properties of ROS are largely due to the reversible oxidation of redox-sensitive target proteins, and especially of protein tyrosine phosphatases, whose activity is dependent on the redox state of a low pKa active site cysteine. A variety of mitogenic signals, including those released by receptor tyrosine kinase (RTKs) ligands and oncogenic H-Ras, involve as a critical downstream event the intracellular generation of ROS. Signaling by integrins is also essential for the growth of most cell types and is constantly integrated with growth factor signaling. We provide here evidence that intracellular ROS are generated after integrin engagement and that these oxidant intermediates are necessary for integrin signaling during fibroblast adhesion and spreading. Moreover, we propose a synergistic action of integrins and RTKs for redox signaling. Integrin-induced ROS are required to oxidize/inhibit the low molecular weight phosphotyrosine phosphatase, thereby preventing the enzyme from dephosphorylating and inactivating FAK. Accordingly, FAK phosphorylation and other downstream events, including MAPK phosphorylation, Src phosphorylation, focal adhesion formation, and cell spreading, are all significantly attenuated by inhibition of redox signaling. Hence, we have outlined a redox circuitry whereby, upon cell adhesion, oxidative inhibition of a protein tyrosine phosphatase promotes the phosphorylation/activation and the downstream signaling of FAK and, as a final event, cell adhesion and spreading onto fibronectin.
Figures













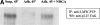
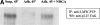
Similar articles
-
Reactive oxygen species as mediators of cell adhesion.Ital J Biochem. 2003 Mar;52(1):28-32. Ital J Biochem. 2003. PMID: 12833635 Review.
-
PTP alpha regulates integrin-stimulated FAK autophosphorylation and cytoskeletal rearrangement in cell spreading and migration.J Cell Biol. 2003 Jan 6;160(1):137-46. doi: 10.1083/jcb.200206049. Epub 2003 Jan 6. J Cell Biol. 2003. PMID: 12515828 Free PMC article.
-
Integrin-dependent PLC-gamma1 phosphorylation mediates fibronectin-dependent adhesion.J Cell Sci. 2005 Feb 1;118(Pt 3):601-10. doi: 10.1242/jcs.01643. Epub 2005 Jan 18. J Cell Sci. 2005. PMID: 15657076
-
Phosphospecific antibodies reveal focal adhesion kinase activation loop phosphorylation in nascent and mature focal adhesions and requirement for the autophosphorylation site.Cell Growth Differ. 2000 Jan;11(1):41-8. Cell Growth Differ. 2000. PMID: 10672902
-
The interplay between Src and integrins in normal and tumor biology.Oncogene. 2004 Oct 18;23(48):7928-46. doi: 10.1038/sj.onc.1208080. Oncogene. 2004. PMID: 15489911 Review.
Cited by
-
Suppression of mitochondrial complex I influences cell metastatic properties.PLoS One. 2013 Apr 22;8(4):e61677. doi: 10.1371/journal.pone.0061677. Print 2013. PLoS One. 2013. PMID: 23630608 Free PMC article.
-
Study of the anti-inflammatory effects of low-dose radiation: The contribution of biphasic regulation of the antioxidative system in endothelial cells.Strahlenther Onkol. 2015 Sep;191(9):742-9. doi: 10.1007/s00066-015-0848-9. Epub 2015 Jun 8. Strahlenther Onkol. 2015. PMID: 26051282
-
Characterization of crude Echis carinatus venom-induced cytotoxicity in HEK 293T cells.J Venom Res. 2011;2:59-67. Epub 2011 Dec 28. J Venom Res. 2011. PMID: 22331993 Free PMC article.
-
Tks5-dependent, nox-mediated generation of reactive oxygen species is necessary for invadopodia formation.Sci Signal. 2009 Sep 15;2(88):ra53. doi: 10.1126/scisignal.2000368. Sci Signal. 2009. PMID: 19755709 Free PMC article.
-
A specific alpha5beta1-integrin conformation promotes directional integrin translocation and fibronectin matrix formation.J Cell Sci. 2005 Jan 15;118(Pt 2):291-300. doi: 10.1242/jcs.01623. Epub 2004 Dec 22. J Cell Sci. 2005. PMID: 15615773 Free PMC article.
References
-
- Abo, A., E. Pick, A. Hall, N. Totty, C.G. Teahan, and A.W. Segal. 1991. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 353:668–670. - PubMed
-
- Bae, Y.S., S.W. Kang, M.S. Seo, I.C. Baines, E. Tekle, P.B. Chock, and S.G. Rhee. 1997. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 272:217–221. - PubMed
-
- Bucciantini, M., P. Chiarugi, P. Cirri, L. Taddei, M. Stefani, G. Raugei, P. Nordlund, and G. Ramponi. 1999. The low Mr phosphotyrosine protein phosphatase behaves differently when phosphorylated at Tyr131 or Tyr132 by Src kinase. FEBS Lett. 456:73–78. - PubMed
-
- Caselli, A., R. Marzocchini, G. Camici, G. Manao, G. Moneti, G. Pieraccini, and G. Ramponi. 1998. The inactivation mechanism of low molecular weight phosphotyrosine-protein phosphatase by H2O2. J. Biol. Chem. 273:32554–32560. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

