Cell adhesion to the extracellular matrix protein fibronectin modulates radiation-dependent G2 phase arrest involving integrin-linked kinase (ILK) and glycogen synthase kinase-3beta (GSK-3beta) in vitro
- PMID: 12778079
- PMCID: PMC2741045
- DOI: 10.1038/sj.bjc.6600912
Cell adhesion to the extracellular matrix protein fibronectin modulates radiation-dependent G2 phase arrest involving integrin-linked kinase (ILK) and glycogen synthase kinase-3beta (GSK-3beta) in vitro
Abstract
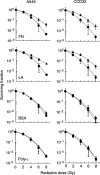
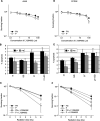
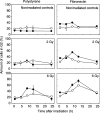
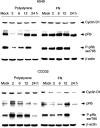
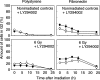
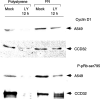
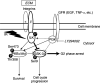
Cell adhesion to extracellular matrix (ECM) is thought to confer resistance against cell-damaging agents, that is, drugs and radiation, in tumour and normal cells in vitro. The dependence of cell survival on beta1-integrin-linked kinase (ILK), protein kinase Balpha/Akt (PKBalpha/Akt) and glycogen synthase kinase-3beta (GSK-3beta) activity, which participate in beta1-integrin signalling and cell cycle progression was investigated as a function of radiation exposure. Colony-formation assays on polystyrene, fibronectin (FN), laminin (LA), bovine serum albumin (BSA) or poly-L-lysine (poly-L) (0-8 Gy), kinase assays, flow cytometric DNA and annexin-V analysis and immunoblotting were performed in nonirradiated and irradiated (2 or 6 Gy) A549 human lung cancer cells and CCD32 normal human lung fibroblasts. Cell contact to FN in contrast to polystyrene elevated basal ILK, PKBalpha/Akt and GSK-3beta kinase activities in A549 and CCD32 cells, as well as the basal amount of A549 G2 phase cells. Irradiation on FN or LA as compared to polystyrene, BSA or poly-L significantly improved cell survival. Following irradiation, kinase activities were stimulated strongly on polystyrene but showed to be less prominent on FN, which was because of the FN-related basal induction. Following irradiation, FN compared to polystyrene enlarged and prolonged G2 arrest in both the cell lines. For the analysis of phosphatidylinositol-3 kinase (PI3-K) dependence of protein kinases and cell cycle transition, the PI3-K inhibitors LY294002 and wortmannin were used showing decreased kinase activities, antiproliferative and radiation-dependent G2 accumulation-abrogating effects accompanied by downregulation of cyclin D1 and phospho-pRb in cells attached to polystyrene. Fibronectin partly abrogated these effects PI3-K-independently. These findings suggest a novel pathway that makes direct phosphorylation of GSK-3beta by ILK feasible after irradiation. Conclusively, the data indicate that ILK, PKBalpha/Akt and GSK-3beta are involved in modulations of the cell cycle after irradiation. These interactions are strictly dependent on ECM components in a cell line-specific manner. Our findings provide molecular insights into mechanisms likely to be important for ECM-dependent cell survival and cellular radioresistance as well as tumour growth.
Figures

Similar articles
-
Arrest of human lung fibroblasts in G2 phase after irradiation is regulated by converging phosphatidylinositol-3 kinase and beta1-integrin signaling in vitro.Int J Radiat Oncol Biol Phys. 2004 Feb 1;58(2):453-62. doi: 10.1016/j.ijrobp.2003.09.069. Int J Radiat Oncol Biol Phys. 2004. PMID: 14751515
-
Fibronectin and laminin increase resistance to ionizing radiation and the cytotoxic drug Ukrain in human tumour and normal cells in vitro.Int J Radiat Biol. 2003 Sep;79(9):709-20. doi: 10.1080/09553000310001610240. Int J Radiat Biol. 2003. PMID: 14703944
-
Ionizing radiation induces up-regulation of functional beta1-integrin in human lung tumour cell lines in vitro.Int J Radiat Biol. 2002 May;78(5):347-57. doi: 10.1080/09553000110117340. Int J Radiat Biol. 2002. PMID: 12020426
-
Cell Cycle Regulation by Integrin-Mediated Adhesion.Cells. 2022 Aug 14;11(16):2521. doi: 10.3390/cells11162521. Cells. 2022. PMID: 36010598 Free PMC article. Review.
-
Cell cycle control by cell-matrix interactions.Curr Opin Cell Biol. 2024 Feb;86:102288. doi: 10.1016/j.ceb.2023.102288. Epub 2023 Dec 5. Curr Opin Cell Biol. 2024. PMID: 38056140 Review.
Cited by
-
Alveolar type II cells from ethanol-fed rats produce a fibronectin-enriched extracellular matrix that promotes monocyte activation.Alcohol. 2007 Aug;41(5):317-24. doi: 10.1016/j.alcohol.2007.04.009. Alcohol. 2007. PMID: 17889308 Free PMC article.
-
Irradiation differentially affects substratum-dependent survival, adhesion, and invasion of glioblastoma cell lines.Br J Cancer. 2003 Dec 1;89(11):2122-32. doi: 10.1038/sj.bjc.6601429. Br J Cancer. 2003. PMID: 14647148 Free PMC article.
-
Identification of Key Genes and Pathways in Female Lung Cancer Patients Who Never Smoked by a Bioinformatics Analysis.J Cancer. 2019 Jan 1;10(1):51-60. doi: 10.7150/jca.26908. eCollection 2019. J Cancer. 2019. PMID: 30662525 Free PMC article.
-
Extracellular Matrix Protein Coating of Processed Fish Scales Improves Human Corneal Endothelial Cell Adhesion and Proliferation.Transl Vis Sci Technol. 2019 May 30;8(3):27. doi: 10.1167/tvst.8.3.27. eCollection 2019 May. Transl Vis Sci Technol. 2019. PMID: 31171994 Free PMC article.
-
Novel robust biomarkers for human bladder cancer based on activation of intracellular signaling pathways.Oncotarget. 2014 Oct 15;5(19):9022-32. doi: 10.18632/oncotarget.2493. Oncotarget. 2014. PMID: 25296972 Free PMC article.
References
-
- Akimoto T, Nonaka T, Ishikawa H, Sakurai H, Saitoh JI, Takahashi T, Mitsuhashi N (2001) Genistein, a tyrosine kinase inhibitor, enhanced radiosensitivity in human esophageal cancer cell lines in vitro : possible involvement of inhibition of survival signal transduction pathways. Int J Radiat Oncol Biol Phys 50: 195–201 - PubMed
-
- Brognard J, Clark AS, Ni Y, Dennis PA (2001) Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res 61: 3986–3997 - PubMed
-
- Chen Q, Kinch MS, Lin TH, Burridge K, Juliano RL (1994) Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem 269: 26602–26605 - PubMed
-
- Cordes N, Blaese MA, Meineke V, van Beuningen D (2002) Ionizing radiation induces up-regulation of functional β1-integrin in lung tumour cell lines in vitro. Int J Radiat Biol 78: 347–357 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

