Negative charges in the transmembrane domains of the HERG K channel are involved in the activation- and deactivation-gating processes
- PMID: 12771194
- PMCID: PMC2217355
- DOI: 10.1085/jgp.200308788
Negative charges in the transmembrane domains of the HERG K channel are involved in the activation- and deactivation-gating processes
Abstract
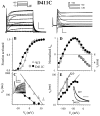
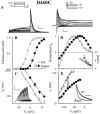
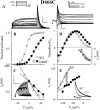
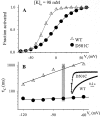
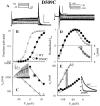
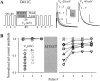
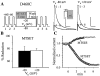
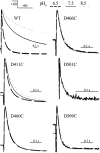
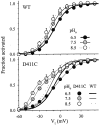
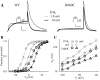
The transmembrane domains of HERG (S1-S3) contain six negative charges: three are conserved in all voltage-gated K channels (D456 and D466 in S2, D501 in S3) and three are unique to the EAG family (D411 in S1, D460 in S2, and D509 in S3). We infer the functional role of these aspartates by studying how substituting them with cysteine, one at a time, affects the channel function. D456C is not functional, suggesting that this negative charge may play a critical role in channel protein folding during biogenesis, as has been shown for its counterpart in the Shaker channel. Data from the other five functional mutants suggest that D411 can stabilize the HERG channel in the closed state, while D460 and D509 have the opposite effect. D466 and D501 both may contribute to voltage-sensing during the activation process. On the other hand, all five aspartates work in a concerted fashion in contributing to the slow deactivation process of the HERG channel. Accessibility tests of the introduced thiol groups to extracellular MTS reagents indicate that water-filled crevices penetrate deep into the HERG protein core, reaching the cytoplasmic halves of S1 and S2. At these deep locations, accessibility of 411C and 466C to the extracellular aqueous phase is voltage dependent, suggesting that conformational changes occur in S1 and S2 or the surrounding crevices during gating. Increasing extracellular [H+] accelerates HERG deactivation. This effect is suppressed by substituting the aspartates with cysteine, suggesting that protonation of these aspartates may contribute to the signaling pathway whereby external [H+] influences conformational changes in the channel's cytoplasmic domains (where deactivation takes place). There is no evidence for a metal ion binding site coordinated by negative charges in the transmembrane domains of HERG, as the one described for the EAG channel.
Figures


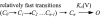




Similar articles
-
Interactions between charged residues in the transmembrane segments of the voltage-sensing domain in the hERG channel.J Membr Biol. 2005 Oct;207(3):169-81. doi: 10.1007/s00232-005-0812-1. J Membr Biol. 2005. PMID: 16550488
-
Differences between ion binding to eag and HERG voltage sensors contribute to differential regulation of activation and deactivation gating.Channels (Austin). 2007 Nov-Dec;1(6):429-37. doi: 10.4161/chan.1.6.5760. Epub 2008 Feb 19. Channels (Austin). 2007. PMID: 18690045 Free PMC article.
-
Gating charges in the activation and inactivation processes of the HERG channel.J Gen Physiol. 2004 Dec;124(6):703-18. doi: 10.1085/jgp.200409119. Epub 2004 Nov 15. J Gen Physiol. 2004. PMID: 15545400 Free PMC article.
-
Structural organization of the voltage sensor in voltage-dependent potassium channels.Novartis Found Symp. 2002;245:178-90; discussion 190-2, 261-4. Novartis Found Symp. 2002. PMID: 12027007 Review.
-
The HERG K+ channel: progress in understanding the molecular basis of its unusual gating kinetics.Eur Biophys J. 2004 Apr;33(2):89-97. doi: 10.1007/s00249-003-0338-3. Epub 2003 Sep 10. Eur Biophys J. 2004. PMID: 13680209 Review.
Cited by
-
Cytoplasmic domains and voltage-dependent potassium channel gating.Front Pharmacol. 2012 Mar 23;3:49. doi: 10.3389/fphar.2012.00049. eCollection 2012. Front Pharmacol. 2012. PMID: 22470342 Free PMC article.
-
Voltage-dependent gating of HERG potassium channels.Front Pharmacol. 2012 May 8;3:83. doi: 10.3389/fphar.2012.00083. eCollection 2012. Front Pharmacol. 2012. PMID: 22586397 Free PMC article.
-
Mechanism of loss of Kv11.1 K+ current in mutant T421M-Kv11.1-expressing rat ventricular myocytes: interaction of trafficking and gating.Circulation. 2012 Dec 11;126(24):2809-18. doi: 10.1161/CIRCULATIONAHA.112.118018. Epub 2012 Nov 6. Circulation. 2012. PMID: 23136156 Free PMC article.
-
Molecular determinants of interactions between the N-terminal domain and the transmembrane core that modulate hERG K+ channel gating.PLoS One. 2011;6(9):e24674. doi: 10.1371/journal.pone.0024674. Epub 2011 Sep 15. PLoS One. 2011. PMID: 21935437 Free PMC article.
-
Molecular mapping of a site for Cd2+-induced modification of human ether-à-go-go-related gene (hERG) channel activation.J Physiol. 2005 Sep 15;567(Pt 3):737-55. doi: 10.1113/jphysiol.2005.089094. Epub 2005 Jun 23. J Physiol. 2005. PMID: 15975984 Free PMC article.

