Promyelocytic leukemia protein mediates interferon-based anti-herpes simplex virus 1 effects
- PMID: 12768029
- PMCID: PMC156157
- DOI: 10.1128/jvi.77.12.7101-7105.2003
Promyelocytic leukemia protein mediates interferon-based anti-herpes simplex virus 1 effects
Abstract
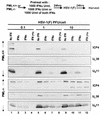
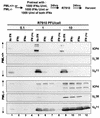
Herpes simplex virus (HSV) 1 disaggregates the nuclear domain 10 (ND10) nuclear structures and disperses its organizing promyelocytic leukemia protein (PML). An earlier report showed that ectopic overexpression of PML precludes the disaggregation of ND10 but has no effect on viral replication. PML has been reported to mediate the effects of interferon (IFN) and viral mutants lacking ICP0 (Delta alpha 0 mutants). To test the hypothesis that HSV disaggregates ND10 structures and disperses PML to preclude IFN-mediated antiviral effects, we tested the accumulation of viral proteins and virus yields from murine PML(+/+) and PML(-/-) cells mock treated or exposed to IFN-alpha, IFN-gamma, or both and infected with the wild-type or Delta alpha 0 mutant virus. We report the following results. (i) The levels of growth of wild-type and mutant viruses and of accumulation of viral proteins were not significantly different in untreated PML(+/+) and PML(-/-) cells. (ii) Major effects of IFN-alpha and -gamma were observed in PML(+/+) cells infected with the Delta alpha 0 mutant virus, and more minor effects were observed in cells infected with the wild-type virus. The effects of the IFNs on either wild-type or the mutant virus in PML(-/-) cells were minimal. (iii) The mixture of IFN-alpha and -gamma was more effective than either IFN alone, but again, the effect was more drastic in PML(+/+) cells than in PML(-/-) cells. We concluded that the anti-HSV state induced by exogenous IFN is mediated by PML and that the virus targets the ND10 structures and disseminates PML in order to preclude the establishment of the antiviral state induced by IFNs.
Figures
Similar articles
-
Overexpression of promyelocytic leukemia protein precludes the dispersal of ND10 structures and has no effect on accumulation of infectious herpes simplex virus 1 or its proteins.J Virol. 2002 Sep;76(18):9355-67. doi: 10.1128/jvi.76.18.9355-9367.2002. J Virol. 2002. PMID: 12186918 Free PMC article.
-
ND10 protein PML is recruited to herpes simplex virus type 1 prereplicative sites and replication compartments in the presence of viral DNA polymerase.J Virol. 1998 Dec;72(12):10100-7. doi: 10.1128/JVI.72.12.10100-10107.1998. J Virol. 1998. PMID: 9811750 Free PMC article.
-
PML plays both inimical and beneficial roles in HSV-1 replication.Proc Natl Acad Sci U S A. 2016 May 24;113(21):E3022-8. doi: 10.1073/pnas.1605513113. Epub 2016 May 9. Proc Natl Acad Sci U S A. 2016. PMID: 27162364 Free PMC article.
-
Role and fate of PML nuclear bodies in response to interferon and viral infections.Oncogene. 2001 Oct 29;20(49):7274-86. doi: 10.1038/sj.onc.1204854. Oncogene. 2001. PMID: 11704856 Review.
-
The use of fluorescence microscopy to study the association between herpesviruses and intrinsic resistance factors.Viruses. 2011 Dec;3(12):2412-24. doi: 10.3390/v3122412. Epub 2011 Dec 7. Viruses. 2011. PMID: 22355446 Free PMC article. Review.
Cited by
-
Cell Intrinsic Determinants of Alpha Herpesvirus Latency and Pathogenesis in the Nervous System.Viruses. 2023 Nov 22;15(12):2284. doi: 10.3390/v15122284. Viruses. 2023. PMID: 38140525 Free PMC article. Review.
-
Network- and enrichment-based inference of phenotypes and targets from large-scale disease maps.NPJ Syst Biol Appl. 2022 Apr 26;8(1):13. doi: 10.1038/s41540-022-00222-z. NPJ Syst Biol Appl. 2022. PMID: 35473910 Free PMC article.
-
Physical requirements and functional consequences of complex formation between the cytomegalovirus IE1 protein and human STAT2.J Virol. 2009 Dec;83(24):12854-70. doi: 10.1128/JVI.01164-09. Epub 2009 Oct 7. J Virol. 2009. PMID: 19812155 Free PMC article.
-
Disruption of PML nuclear bodies is mediated by ORF61 SUMO-interacting motifs and required for varicella-zoster virus pathogenesis in skin.PLoS Pathog. 2011 Aug;7(8):e1002157. doi: 10.1371/journal.ppat.1002157. Epub 2011 Aug 25. PLoS Pathog. 2011. PMID: 21901090 Free PMC article.
-
Cellular proteins PML and Daxx mediate an innate antiviral defense antagonized by the adenovirus E4 ORF3 protein.J Virol. 2008 Aug;82(15):7325-35. doi: 10.1128/JVI.00723-08. Epub 2008 May 14. J Virol. 2008. PMID: 18480450 Free PMC article.
References
-
- Ahn, J. H., E. J. Brignole III, and G. S. Hayward. 1998. Disruption of PML subnuclear domains by the acidic IE1 protein of human cytomegalovirus is mediated through interaction with PML and may modulate a RING finger-dependent cryptic transactivator function of PML. Mol. Cell. Biol. 18:4899-4913. - PMC - PubMed
-
- Doucas, V., A. M. Ishov, A. Romo, H. Juguilon, M. D. Weitzman, R. M. Evans, and G. G. Maul. 1996. Adenovirus replication is coupled with the dynamic properties of the PML nuclear structure. Genes Dev. 10:196-207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA78766/CA/NCI NIH HHS/United States
- CA83939/CA/NCI NIH HHS/United States
- R01 CA078766/CA/NCI NIH HHS/United States
- CA87661/CA/NCI NIH HHS/United States
- R01 CA071692/CA/NCI NIH HHS/United States
- P01 CA087661/CA/NCI NIH HHS/United States
- R37 CA071692/CA/NCI NIH HHS/United States
- P01 CA071933/CA/NCI NIH HHS/United States
- R01 CA088860/CA/NCI NIH HHS/United States
- CA88860/CA/NCI NIH HHS/United States
- R37 CA078766/CA/NCI NIH HHS/United States
- R01 CA083939/CA/NCI NIH HHS/United States
- CA71692/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

