HDL-associated estradiol stimulates endothelial NO synthase and vasodilation in an SR-BI-dependent manner
- PMID: 12750408
- PMCID: PMC155043
- DOI: 10.1172/JCI16777
HDL-associated estradiol stimulates endothelial NO synthase and vasodilation in an SR-BI-dependent manner
Abstract
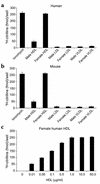
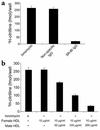
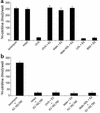
Cardiovascular diseases remain the leading cause of death in the United States. Two factors associated with a decreased risk of developing cardiovascular disease are elevated HDL levels and sex - specifically, a decreased risk is found in premenopausal women. HDL and estrogen stimulate eNOS and the production of nitric oxide, which has numerous protective effects in the vascular system including vasodilation, antiadhesion, and anti-inflammatory effects. We tested the hypothesis that HDL binds to its receptor, scavenger receptor class B type I (SR-BI), and delivers estrogen to eNOS, thereby stimulating the enzyme. HDL isolated from women stimulated eNOS, whereas HDL isolated from men had minimal activity. Studies with ovariectomized and ovariectomized/estrogen replacement mouse models demonstrated that HDL-associated estradiol stimulation of eNOS is SR-BI dependent. Furthermore, female HDL, but not male HDL, promoted the relaxation of muscle strips isolated from C57BL/6 mice but not SR-BI null mice. Finally, HDL isolated from premenopausal women or postmenopausal women receiving estradiol replacement therapy stimulated eNOS, whereas HDL isolated from postmenopausal women did not stimulate eNOS. We conclude that HDL-associated estrodial is capable of the stimulating eNOS. These studies establish a new paradigm for examining the cardiovascular effects of HDL and estrogen.
Figures



Similar articles
-
High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase.Nat Med. 2001 Jul;7(7):853-7. doi: 10.1038/89986. Nat Med. 2001. PMID: 11433352
-
A novel ligand-independent apoptotic pathway induced by scavenger receptor class B, type I and suppressed by endothelial nitric-oxide synthase and high density lipoprotein.J Biol Chem. 2005 May 13;280(19):19087-96. doi: 10.1074/jbc.M500944200. Epub 2005 Mar 4. J Biol Chem. 2005. PMID: 15749707
-
High density lipoprotein binding to scavenger receptor, Class B, type I activates endothelial nitric-oxide synthase in a ceramide-dependent manner.J Biol Chem. 2002 Mar 29;277(13):11058-63. doi: 10.1074/jbc.M110985200. Epub 2002 Jan 15. J Biol Chem. 2002. PMID: 11792700
-
HDL stimulation of endothelial nitric oxide synthase: a novel mechanism of HDL action.Trends Cardiovasc Med. 2003 Aug;13(6):226-31. doi: 10.1016/s1050-1738(03)00098-7. Trends Cardiovasc Med. 2003. PMID: 12922018 Review.
-
Influence of the HDL receptor SR-BI on lipoprotein metabolism and atherosclerosis.Arterioscler Thromb Vasc Biol. 2003 Oct 1;23(10):1732-8. doi: 10.1161/01.ATV.0000091363.28501.84. Epub 2003 Aug 14. Arterioscler Thromb Vasc Biol. 2003. PMID: 12920050 Review.
Cited by
-
Cholesterol binding, efflux, and a PDZ-interacting domain of scavenger receptor-BI mediate HDL-initiated signaling.J Clin Invest. 2005 Apr;115(4):969-77. doi: 10.1172/JCI23858. Epub 2005 Mar 24. J Clin Invest. 2005. PMID: 15841181 Free PMC article.
-
SR-BI (Scavenger Receptor Class B Type 1) Is Critical in Maintaining Normal T-Cell Development and Enhancing Thymic Regeneration.Arterioscler Thromb Vasc Biol. 2018 Nov;38(11):2706-2717. doi: 10.1161/ATVBAHA.118.311728. Arterioscler Thromb Vasc Biol. 2018. PMID: 30354229 Free PMC article.
-
Scavenger receptor class B type I and immune dysfunctions.Curr Opin Endocrinol Diabetes Obes. 2014 Apr;21(2):121-8. doi: 10.1097/MED.0000000000000046. Curr Opin Endocrinol Diabetes Obes. 2014. PMID: 24569553 Free PMC article. Review.
-
Estradiol and HDL Function in Women - A Partnership for Life.J Clin Endocrinol Metab. 2022 Apr 19;107(5):e2192-e2194. doi: 10.1210/clinem/dgab811. J Clin Endocrinol Metab. 2022. PMID: 34788853 Free PMC article. No abstract available.
-
High-Density Lipoprotein-Associated Paraoxonase-1 (PON-1) and Scavenger Receptor Class B Type 1 (SRB-1) in Coronary Artery Disease: Correlation with Disease Severity.J Clin Med. 2024 Sep 15;13(18):5480. doi: 10.3390/jcm13185480. J Clin Med. 2024. PMID: 39336967 Free PMC article.
References
-
- Mendelsohn ME. Protective effects of estrogen on the cardiovascular system. Am. J. Cardiol. 2002;89:12E–17E. - PubMed
-
- Mendelsohn ME. Mechanisms of estrogen action in the cardiovascular system. J. Steroid Biochem. 2000;74:337–343. - PubMed
-
- von Eckardstein A, Assmann G. Prevention of coronary heart disease by raising high-density lipoprotein cholesterol. Curr. Opin. Lipidol. 2000;11:627–637. - PubMed
-
- Yuhanna IS, et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med. 2001;7:853–857. - PubMed
-
- Li X-A, et al. High-density lipoprotein binding to scavenger receptor class B, type I activates endothelial nitric oxide synthase in a ceramide-dependent manner. J. Biol. Chem. 2002;277:11058–11063. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

