Role of the reversible xanthophyll cycle in the photosystem II damage and repair cycle in Dunaliella salina
- PMID: 12746540
- PMCID: PMC166980
- DOI: 10.1104/pp.102.019620
Role of the reversible xanthophyll cycle in the photosystem II damage and repair cycle in Dunaliella salina
Abstract
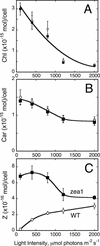
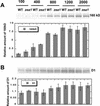
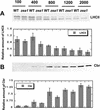
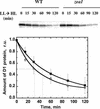
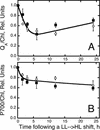
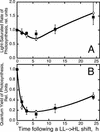
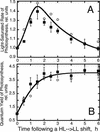
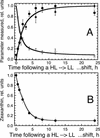
The Dunaliella salina photosynthetic apparatus organization and function was investigated in wild type (WT) and a mutant (zea1) lacking all beta,beta-epoxycarotenoids derived from zeaxanthin (Z). The zea1 mutant lacked antheraxanthin, violaxanthin, and neoxanthin from its thylakoid membranes but constitutively accumulated Z instead. It also lacked the so-called xanthophyll cycle, which, upon irradiance stress, reversibly converts violaxanthin to Z via a de-epoxidation reaction. Despite the pronounced difference observed in the composition of beta,beta-epoxycarotenoids between WT and zea1, no discernible difference could be observed between the two strains in terms of growth, photosynthesis, organization of the photosynthetic apparatus, photo-acclimation, sensitivity to photodamage, or recovery from photo-inhibition. WT and zea1 were probed for the above parameters over a broad range of growth irradiance and upon light shift experiments (low light to high light shift and vice versa). A constitutive accumulation of Z in the zea1 strain did not affect the acclimation of the photosynthetic apparatus to irradiance, as evidenced by indistinguishable irradiance-dependent adjustments in the chlorophyll antenna size and photosystem content of WT and zea1 strain. In addition, a constitutive accumulation of Z in the zea1 strain did not affect rates of photodamage or the recovery of the photosynthetic apparatus from photo-inhibition. However, Z in the WT accumulated in parallel with the accumulation of photodamaged PSII centers in the chloroplast thylakoids and decayed in tandem with a chloroplast recovery from photo-inhibition. These results suggest a role for Z in the protection of photodamaged and disassembled PSII reaction centers, apparently needed while PSII is in the process of degradation and replacement of the D1/32-kD reaction center protein.
Figures

Similar articles
-
Involvement of zeaxanthin and of the Cbr protein in the repair of photosystem II from photoinhibition in the green alga Dunaliella salina.Biochim Biophys Acta. 2001 Nov 1;1506(3):244-59. doi: 10.1016/s0005-2728(01)00223-7. Biochim Biophys Acta. 2001. PMID: 11779558
-
Biosynthesis and distribution of chlorophyll among the photosystems during recovery of the green alga Dunaliella salina from irradiance stress.Plant Physiol. 2002 Feb;128(2):603-14. doi: 10.1104/pp.010595. Plant Physiol. 2002. PMID: 11842163 Free PMC article.
-
Absence of lutein, violaxanthin and neoxanthin affects the functional chlorophyll antenna size of photosystem-II but not that of photosystem-I in the green alga Chlamydomonas reinhardtii.Plant Cell Physiol. 2001 May;42(5):482-91. doi: 10.1093/pcp/pce058. Plant Cell Physiol. 2001. PMID: 11382814
-
Carotenoids, versatile components of oxygenic photosynthesis.Prog Lipid Res. 2013 Oct;52(4):539-61. doi: 10.1016/j.plipres.2013.07.001. Epub 2013 Jul 26. Prog Lipid Res. 2013. PMID: 23896007 Review.
-
Light-harvesting regulation from leaf to molecule with the emphasis on rapid changes in antenna size.Photosynth Res. 2015 May;124(2):137-58. doi: 10.1007/s11120-015-0115-z. Epub 2015 Mar 14. Photosynth Res. 2015. PMID: 25773873 Review.
Cited by
-
Light- and pH-dependent structural changes in the PsbS subunit of photosystem II.Proc Natl Acad Sci U S A. 2003 Dec 9;100(25):15265-70. doi: 10.1073/pnas.2533072100. Epub 2003 Dec 1. Proc Natl Acad Sci U S A. 2003. PMID: 14657329 Free PMC article.
-
Development of a Dunaliella tertiolecta Strain with Increased Zeaxanthin Content Using Random Mutagenesis.Mar Drugs. 2017 Jun 21;15(6):189. doi: 10.3390/md15060189. Mar Drugs. 2017. PMID: 28635662 Free PMC article.
-
Cloning and expression study of a putative carotene biosynthesis related (cbr) gene from the halotolerant green alga Dunaliella salina.Mol Biol Rep. 2008 Sep;35(3):321-7. doi: 10.1007/s11033-007-9089-z. Epub 2007 Jun 12. Mol Biol Rep. 2008. PMID: 17562223
-
Morpho-Physiological Traits and Oil Quality in Drought-Tolerant Raphanus sativus L. Used for Biofuel Production.Plants (Basel). 2024 Jun 7;13(12):1583. doi: 10.3390/plants13121583. Plants (Basel). 2024. PMID: 38931015 Free PMC article.
-
LPA2 protein is involved in photosystem II assembly in Chlamydomonas reinhardtii.Plant J. 2021 Sep;107(6):1648-1662. doi: 10.1111/tpj.15405. Epub 2021 Jul 31. Plant J. 2021. PMID: 34218480 Free PMC article.
References
-
- Anderson JM. Photoregulation of the composition, function and structure of thylakoid membranes. Annu Rev Plant Physiol. 1986;37:93–136.
-
- Aro EM, Virgin I, Andersson B. Photoinhibition of photosystem II: Inactivation, protein damage and turnover. Biochim Biophys Acta. 1993;1143:113–134. - PubMed
-
- Banet G, Pick U, Zamir A. Light-harvesting complex II pigments and proteins in association with Cbr, a homologue of higher-plant early light-inducible proteins in the unicellular green alga Dunaliella. Planta. 2000;210:947–955. - PubMed
-
- Baroli I, Melis A. Photoinhibition and repair in Dunaliella salina acclimated to different growth irradiances. Planta. 1996;198:640–646. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

