The spatiotemporal expression pattern of the bone morphogenetic protein family in rat ovary cell types during the estrous cycle
- PMID: 12741959
- PMCID: PMC153494
- DOI: 10.1186/1477-7827-1-9
The spatiotemporal expression pattern of the bone morphogenetic protein family in rat ovary cell types during the estrous cycle
Abstract
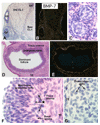
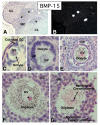
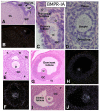
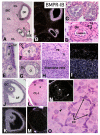
In the mammalian ovary, great interest in the expression and function of the bone morphogenetic protein (BMP) family has been recently generated from evidence of their critical role in determining folliculogenesis and female fertility. Despite extensive work, there is a need to understand the cellular sites of expression of these important regulatory molecules, and how their gene expression changes within the basic ovary cell types through the cycle. Here we have performed a detailed in situ hybridization analysis of the spatial and temporal expression patterns of the BMP ligands (BMP-2, -3, -3b, -4, -6, -7, -15), receptors (BMPR-IA, -IB, -II), and BMP antagonist, follistatin, in rat ovaries over the normal estrous cycle. We have found that: i) all of the mRNAs are expressed in a cell-specific manner in the major classes of ovary cell types (oocyte, granulosa, theca interstitial, theca externa, corpora lutea, secondary interstitial, vascular and ovary surface epithelium); and ii) most undergo dynamic changes during follicular and corpora luteal morphogenesis and histogenesis. The general principle to emerge from these studies is that the developmental programs of folliculogenesis (recruitment, selection, atresia), ovulation, and luteogenesis (luteinization, luteolysis) are accompanied by rather dramatic spatial and temporal changes in the expression patterns of these BMP genes. These results lead us to hypothesize previously unanticipated roles for the BMP family in determining fundamental developmental events that ensure the proper timing and developmental events required for the generation of the estrous cycle.
Figures
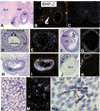
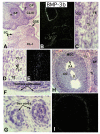
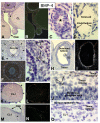
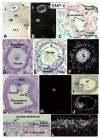

Similar articles
-
Analysis of spatial and temporal expression patterns of bone morphogenetic protein family members in the rat uterus over the estrous cycle.J Endocrinol. 2004 Aug;182(2):203-17. doi: 10.1677/joe.0.1820203. J Endocrinol. 2004. PMID: 15283681
-
Expression of bone morphogenetic protein2 (BMP2), BMP4 and BMP receptors in the bovine ovary but absence of effects of BMP2 and BMP4 during IVM on bovine oocyte nuclear maturation and subsequent embryo development.Theriogenology. 2005 Feb;63(3):872-89. doi: 10.1016/j.theriogenology.2004.05.013. Theriogenology. 2005. PMID: 15629804
-
BMPs and BMPRs in chicken ovary and effects of BMP-4 and -7 on granulosa cell proliferation and progesterone production in vitro.Am J Physiol Endocrinol Metab. 2003 Nov;285(5):E973-83. doi: 10.1152/ajpendo.00104.2003. Epub 2003 Jul 29. Am J Physiol Endocrinol Metab. 2003. PMID: 12888485
-
The role of bone morphogenetic proteins in ovarian function.Reprod Suppl. 2003;61:323-37. Reprod Suppl. 2003. PMID: 14635945 Review.
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
Cited by
-
Fibrillins in adult human ovary and polycystic ovary syndrome: is fibrillin-3 affected in PCOS?J Histochem Cytochem. 2010 Oct;58(10):903-15. doi: 10.1369/jhc.2010.956615. J Histochem Cytochem. 2010. PMID: 20855553 Free PMC article.
-
Immune physiology in tissue regeneration and aging, tumor growth, and regenerative medicine.Aging (Albany NY). 2009 Feb 13;1(2):157-81. doi: 10.18632/aging.100024. Aging (Albany NY). 2009. PMID: 20195382 Free PMC article. Review.
-
Ligand-receptor promiscuity enables cellular addressing.Cell Syst. 2022 May 18;13(5):408-425.e12. doi: 10.1016/j.cels.2022.03.001. Epub 2022 Apr 13. Cell Syst. 2022. PMID: 35421362 Free PMC article.
-
Bone morphogenetic protein signaling transcription factor (SMAD) function in granulosa cells.Mol Cell Endocrinol. 2012 Jun 5;356(1-2):40-7. doi: 10.1016/j.mce.2011.06.021. Epub 2011 Jul 7. Mol Cell Endocrinol. 2012. PMID: 21763749 Free PMC article. Review.
-
The role of FSH and TGF-β superfamily in follicle atresia.Aging (Albany NY). 2018 Mar 2;10(3):305-321. doi: 10.18632/aging.101391. Aging (Albany NY). 2018. PMID: 29500332 Free PMC article. Review.
References
-
- Shimasaki S, Moore RK, Erickson GF, Otsuka F. The role of bone morphogenetic proteins in ovarian function. Reproduction. - PubMed
-
- Lyons KM, Pelton RW, Hogan BL. Patterns of expression of murine Vgr-1 and BMP-2a RNA suggest that transforming growth factor-β-like genes coordinately regulate aspects of embryonic development. Genes Dev. 1989;3:1657–1668. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

