Wnt/Wingless signaling through beta-catenin requires the function of both LRP/Arrow and frizzled classes of receptors
- PMID: 12729465
- PMCID: PMC156895
- DOI: 10.1186/1471-2121-4-4
Wnt/Wingless signaling through beta-catenin requires the function of both LRP/Arrow and frizzled classes of receptors
Abstract
Background: Wnt/Wingless (Wg) signals are transduced by seven-transmembrane Frizzleds (Fzs) and the single-transmembrane LDL-receptor-related proteins 5 or 6 (LRP5/6) or Arrow. The aminotermini of LRP and Fz were reported to associate only in the presence of Wnt, implying that Wnt ligands form a trimeric complex with two different receptors. However, it was recently reported that LRPs activate the Wnt/beta-catenin pathway by binding to Axin in a Dishevelled--independent manner, while Fzs transduce Wnt signals through Dishevelled to stabilize beta-catenin. Thus, it is possible that Wnt proteins form separate complexes with Fzs and LRPs, transducing Wnt signals separately, but converging downstream in the Wnt/beta-catenin pathway. The question then arises whether both receptors are absolutely required to transduce Wnt signals.
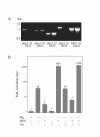
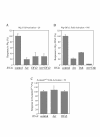
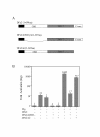
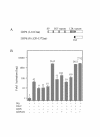
Results: We have established a sensitive luciferase reporter assay in Drosophila S2 cells to determine the level of Wg--stimulated signaling. We demonstrate here that Wg can synergize with DFz2 and function cooperatively with LRP to activate the beta-catenin/Armadillo signaling pathway. Double-strand RNA interference that disrupts the synthesis of either receptor type dramatically impairs Wg signaling activity. Importantly, the pronounced synergistic effect of adding Wg and DFz2 is dependent on Arrow and Dishevelled. The synergy requires the cysteine-rich extracellular domain of DFz2, but not its carboxyterminus. Finally, mammalian LRP6 and its activated forms, which lack most of the extracellular domain of the protein, can activate the Wg signaling pathway and cooperate with Wg and DFz2 in S2 cells. We also show that the aminoterminus of LRP/Arr is required for the synergy between Wg and DFz2.
Conclusion: Our study indicates that Wg signal transduction in S2 cells depends on the function of both LRPs and DFz2, and the results are consistent with the proposal that Wnt/Wg signals through the aminoterminal domains of its dual receptors, activating target genes through Dishevelled.
Figures
Similar articles
-
Truncated mutants of the putative Wnt receptor LRP6/Arrow can stabilize beta-catenin independently of Frizzled proteins.Oncogene. 2004 Jun 17;23(28):4873-84. doi: 10.1038/sj.onc.1207642. Oncogene. 2004. PMID: 15064719 Free PMC article.
-
Wnt signals across the plasma membrane to activate the beta-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP.Development. 2004 Oct;131(20):5103-15. doi: 10.1242/dev.01318. Development. 2004. PMID: 15459103
-
Pathway specificity by the bifunctional receptor frizzled is determined by affinity for wingless.Mol Cell. 2000 Jul;6(1):117-26. Mol Cell. 2000. PMID: 10949033
-
LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way.Development. 2004 Apr;131(8):1663-77. doi: 10.1242/dev.01117. Development. 2004. PMID: 15084453 Review.
-
Wnt signaling: complexity at the surface.J Cell Sci. 2006 Feb 1;119(Pt 3):395-402. doi: 10.1242/jcs.02826. J Cell Sci. 2006. PMID: 16443747 Review.
Cited by
-
The role of the cysteine-rich domain of Frizzled in Wingless-Armadillo signaling.EMBO J. 2005 Oct 5;24(19):3493-503. doi: 10.1038/sj.emboj.7600817. Epub 2005 Sep 15. EMBO J. 2005. PMID: 16163385 Free PMC article.
-
Impact of energy restriction during late gestation on the muscle and blood transcriptome of beef calves after preconditioning.BMC Genomics. 2018 Sep 25;19(1):702. doi: 10.1186/s12864-018-5089-8. BMC Genomics. 2018. PMID: 30253751 Free PMC article.
-
Truncated mutants of the putative Wnt receptor LRP6/Arrow can stabilize beta-catenin independently of Frizzled proteins.Oncogene. 2004 Jun 17;23(28):4873-84. doi: 10.1038/sj.onc.1207642. Oncogene. 2004. PMID: 15064719 Free PMC article.
-
R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6.Proc Natl Acad Sci U S A. 2007 Sep 11;104(37):14700-5. doi: 10.1073/pnas.0702305104. Epub 2007 Sep 5. Proc Natl Acad Sci U S A. 2007. PMID: 17804805 Free PMC article.
-
LRP6 enhances glucose metabolism by promoting TCF7L2-dependent insulin receptor expression and IGF receptor stabilization in humans.Cell Metab. 2013 Feb 5;17(2):197-209. doi: 10.1016/j.cmet.2013.01.009. Cell Metab. 2013. PMID: 23395167 Free PMC article.
References
-
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes & Development. 1997;11:3286–305. - PubMed
-
- Nusse R, Varmus HE. Wnt genes. Cell. 1992;69:1073–87. - PubMed
-
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–51. - PubMed
-
- Yang-Snyder J, Miller JR, Brown JD, Lai CJ, Moon RT. A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr Biol. 1996;6:1302–6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

